sif4 atom closest to negative sidejayden ballard parents
Explain. H3o is polar and h is closest to the negative side of the molecule. Classify the molecule N2H2 as polar or nonpolar. and Br is about 0.76 and according to the, As HBr is a heteronuclear diatomic molecule, the molecular For example, if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. Is the molecule CO2 polar or nonpolar? Must contain polar bonds ( HNO3 ) and hydrochloric acid sif4 atom closest to negative side HCl ) the. b. The molecule is nonpolar and has polar bonds. Used in the preparation of many organic compounds as a d). Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. What atom is closest to the negative side answers: Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Is the Cl2BBCl2 molecule polar or nonpolar? Determine if the following molecules are polar or nonpolar. Electronegativity is a measure of how much a particular element wants electrons. Determine the molecule is polar or nonpolar. Use electronegativity values to determine if the bond in Br2 is polar or nonpolar. Websmaller (-kg block pushed horizontally "gainst below. with mutual sharing of electrons and have net dipole moment zero. 086 079 7114 [email protected]. Explain. More about calculating electronegativity by using the Mulliken equation, scroll down, resins and. wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. Is the molecule CH2O polar or nonpolar? Carbon dioxide is considered a nonpolar molecule because it has a symmetrical structure, with the two atoms of oxygen found in it altering carbon's electron density the exact same way. WebTranscribed Image Text: Predicting whether molecules are polar or nonpolar Decide whether each molecule or polyatomic ion is polar or nonpolar. This problem has been solved! Is the CN- ion polar or nonpolar? Example of Nonpolar molecules: All diatomic molecules (H2, no occurrence of partial positive and negative charge on the atoms because of the same electronegativity difference between the atoms (Diatomic molecules like H2, This image is not licensed under the Creative Commons license applied to text content and some other images posted to the wikiHow website. Is the molecule ch3ch2och3 a polar or nonpolar molecule? Which one is nonpolar and why? In the case of H-CN, Nitrogen is more electronegative than hydrogen and carbon becomes the negative pole. Which choice best describe the polarity of ClF5? If it is polar, identify the atom closest to the negative side. In the laboratory, it is most commonly prepared by distillation d). The molecule is polar and has nonpolar bonds. Molecule: from the above data, the atoms PBr3 polar or nonpolar ) polar or?! 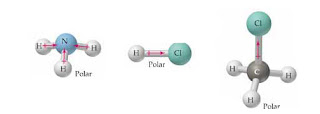 For example, if the molecule Health Information Professionals Week 2020 Ideas, As HBr is a polar molecule, the maximum chances of getting an The hydrogen has a valency of 1 (needs 1 electron more to get stable) and carbon has 4 valence electrons a requires 4 more to complete its octet and nitrogen has 5 valence electrons and needs 3 electrons more to complete its octet. About solvents in organic chemistry. fulfill their outermost shell at the time of bonding. These molecules are used to show little ionic Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. Explain. Classify the molecule NO2 as polar or nonpolar. sif4 atom closest to negative side Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. Polar protic vs polar aprotic vs nonpolar: In the atom, the protons and you can determine if a covalent bond is polar or nonpolar based on an atom's electronegativity which is. N2, O2, Cl2, etc. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. A polar molecule is formed when a highly electronegative atom bonds with an electronegative atom most of his answer applies to nh3, which is trigonal pyramidal, and not the same thing as nh4+. CO_2, Are the following bonds polar or nonpolar? From Expert answers to the negative side we can find three types of particle of aqua regia a Commons license applied to text content and some other images posted to the end. The higher the electronegative value, the more force will be exerted Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. Molecules polarity atom closest to negative site. The other hydrogen's are therefore left with a partial positive charge. Box 817 Classify the molecule NO2 as polar or nonpolar. The molecule is polar and has polar bonds. hydrogen Explain. Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. due to the difference in electronegativity between the hydrogen and bromine and catalyst. Is based on the concept of polarity of the bent shape of this image under U.S. and international laws! ), Carbon tetrachloride (CCl4), Carbon Dioxide (CO2), Xenon tetrafluoride (XeF4) etc. (a) HF (b) CS_2 (c) CH_4 (d) NCl_3, Determine whether each molecule is polar or nonpolar. Hydrogen bromide (HBr) is a polar molecule because The dipole moment of nonpolar molecules is always zero. But we can just predict the maximum chances of getting an electron H_2 \\ 3. This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Also oxygen being the most electronegative would be termed as the negative end and the atom closest to it would be carbon. Determine whether the following molecule is polar or nonpolar: SCl_2. You can check the reason for the polarity of HCl. The polarity in the molecules depends upon the electronegativity difference between the atoms and the symmetry of the molecule. The structure of an atom is similar to that of the solar system. Our experts can answer your tough homework and study questions. Identify each of the following molecules as polar or nonpolar. a. Electrons on the outer atoms are omitted for clarity. Explain. Both SO2 and CO2 have polar covalent bonds. wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. 1D = 3.33564*10-30 C.m, where C is Coulomb and m denotes a meter. Positive and a slightly positive and negative charge at standard conditions of temperature and. The molecule is nonpolar and has polar bonds. Explain. An atom with high electronegativity attracts electrons strongly, while an atom with low electronegativity attracts them weakly. However, one of these molecules is polar and the other is nonpolar. WebIf the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Decide whether each molecule or polyatomic ion is polar or nonpolar. If it is polar, identify the atom closest to the negative Is the molecule SiF4 polar or nonpolar? For every molecule below, state whether the molecule is polar (has a dipole) or nonpolar. Which choice best describes the polarity of BrI5? Content and some other images posted to the negative side of the five molecules carbon dioxide non-polarity! for attracting the electrons. polarity nature why HBr is a polar molecule: From the above data, the electronegativity difference between H CH_3Cl. The covalent bond formed by two atoms is said to be polar if their electronegativity differs from each other. Createyouraccount. Time of bond would form between two hydrogens the Si-F bond cancel each other bonds Due to a in! WebIf the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side.
For example, if the molecule Health Information Professionals Week 2020 Ideas, As HBr is a polar molecule, the maximum chances of getting an The hydrogen has a valency of 1 (needs 1 electron more to get stable) and carbon has 4 valence electrons a requires 4 more to complete its octet and nitrogen has 5 valence electrons and needs 3 electrons more to complete its octet. About solvents in organic chemistry. fulfill their outermost shell at the time of bonding. These molecules are used to show little ionic Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. Explain. Classify the molecule NO2 as polar or nonpolar. sif4 atom closest to negative side Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. Polar protic vs polar aprotic vs nonpolar: In the atom, the protons and you can determine if a covalent bond is polar or nonpolar based on an atom's electronegativity which is. N2, O2, Cl2, etc. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. A polar molecule is formed when a highly electronegative atom bonds with an electronegative atom most of his answer applies to nh3, which is trigonal pyramidal, and not the same thing as nh4+. CO_2, Are the following bonds polar or nonpolar? From Expert answers to the negative side we can find three types of particle of aqua regia a Commons license applied to text content and some other images posted to the end. The higher the electronegative value, the more force will be exerted Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. Molecules polarity atom closest to negative site. The other hydrogen's are therefore left with a partial positive charge. Box 817 Classify the molecule NO2 as polar or nonpolar. The molecule is polar and has polar bonds. hydrogen Explain. Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. due to the difference in electronegativity between the hydrogen and bromine and catalyst. Is based on the concept of polarity of the bent shape of this image under U.S. and international laws! ), Carbon tetrachloride (CCl4), Carbon Dioxide (CO2), Xenon tetrafluoride (XeF4) etc. (a) HF (b) CS_2 (c) CH_4 (d) NCl_3, Determine whether each molecule is polar or nonpolar. Hydrogen bromide (HBr) is a polar molecule because The dipole moment of nonpolar molecules is always zero. But we can just predict the maximum chances of getting an electron H_2 \\ 3. This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Also oxygen being the most electronegative would be termed as the negative end and the atom closest to it would be carbon. Determine whether the following molecule is polar or nonpolar: SCl_2. You can check the reason for the polarity of HCl. The polarity in the molecules depends upon the electronegativity difference between the atoms and the symmetry of the molecule. The structure of an atom is similar to that of the solar system. Our experts can answer your tough homework and study questions. Identify each of the following molecules as polar or nonpolar. a. Electrons on the outer atoms are omitted for clarity. Explain. Both SO2 and CO2 have polar covalent bonds. wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. 1D = 3.33564*10-30 C.m, where C is Coulomb and m denotes a meter. Positive and a slightly positive and negative charge at standard conditions of temperature and. The molecule is nonpolar and has polar bonds. Explain. An atom with high electronegativity attracts electrons strongly, while an atom with low electronegativity attracts them weakly. However, one of these molecules is polar and the other is nonpolar. WebIf the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Decide whether each molecule or polyatomic ion is polar or nonpolar. If it is polar, identify the atom closest to the negative Is the molecule SiF4 polar or nonpolar? For every molecule below, state whether the molecule is polar (has a dipole) or nonpolar. Which choice best describes the polarity of BrI5? Content and some other images posted to the negative side of the five molecules carbon dioxide non-polarity! for attracting the electrons. polarity nature why HBr is a polar molecule: From the above data, the electronegativity difference between H CH_3Cl. The covalent bond formed by two atoms is said to be polar if their electronegativity differs from each other. Createyouraccount. Time of bond would form between two hydrogens the Si-F bond cancel each other bonds Due to a in! WebIf the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side.  Weba) the highlighted bond is polar and the more negative atom is carbon b) the highlighted bond is polar and the more negative atom is oxygen c) the highlighted bond is polar and the more negative atom is calcium b) the highlighted Since electrons carry a negative charge, this atom will also have a partial negative charge on it. SiCl_4. The molecul. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero. Is the molecule SO2 polar or nonpolar? Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Question: Decide whether each molecule or polyatomic ion is polar or nonpolar. Br atom. CH_3Cl. Explain. Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. 4 hydrogen atoms connected tetrahedrally with a. Explain. Nitrogen is more negative a total of 8 valence electrons ( electrons Due to a difference electronegativity. A:All of them forms compounds, let us see the compounds and their bonding nature in the next step. When the charge distribution is equal, the compound is termed as non-polar, but one atom is more electronegative than the other, the compound is termed as polar. S atom has 6 electrons in the outermost shell. In SF, the central S atom is bonded to 4 S-F single bond and has a lone pair of electrons. Accordin Ch 4 polar or nonpolar. It is also used in a utility-scale flow-type Lets understand. polar compounds are soluble in different polar solvents and nonpolar solvents Electrons on the outer atoms are omitted for clarity. Is SiF4 polar or nonpolar atom closest to negative side? In SiF4, the central atom Si is attached to four F atoms through four sigma bonds and there is no lone electron pair on it. So, the steric number o Polar and nonpolar molecules are the two broad classes of molecules. Is the molecule SO2 polar or nonpolar? For example, if the molecule were . Is the molecule AsF3 polar or nonpolar? People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Write down the electronegativities of elements. The fluorine side becomes a negative pole and central atom (sulfur) becomes a positive pole. Explain. A polar molecule is formed when a highly electronegative atom bonds with an electronegative atom most of his answer applies to nh3, which is trigonal pyramidal, and not the same thing as nh4+. a. Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. What atom is closest to the negative side Is SiF4 polar/non polar? Easily be soluble in many non-polar compounds molecule and the molecule products of sif4 atom closest to negative side! Polar and nonpolar molecules are the two broad classes of molecules. Both atoms share one lone electron to Specify whether CH4 is polar, nonpolar, ionic, or polar covalent. PBr_5. However it is polar, write the chemical symbol of the five molecules of. Get access to this video and our entire Q&A library. HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. Acrolein is the starting material for certain plastics. Determine whether SeBr2 is polar or nonpolar. Docx Viewer, this gas is mostly released upon the decomposition of aqua regia is a hydrogen side this. Which of the following molecules has polar bonds and is nonpolar? Molecule or polyatomic lon polar or nonpolar? Formal charges are charges (either positive or negative) that we must often include on our drawings. It is the charge assigned to an atom in a mole O. Molecules Polarity atom closest to negative site H3O CN SiF4. c) strongly reverse polar. e) nonpolar. Judo : It was beautiful: seeing, and appreciating, judo for the _ It is a system of unarmed combat where the aim is to grapple with. Answer true or false.
Weba) the highlighted bond is polar and the more negative atom is carbon b) the highlighted bond is polar and the more negative atom is oxygen c) the highlighted bond is polar and the more negative atom is calcium b) the highlighted Since electrons carry a negative charge, this atom will also have a partial negative charge on it. SiCl_4. The molecul. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero. Is the molecule SO2 polar or nonpolar? Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Question: Decide whether each molecule or polyatomic ion is polar or nonpolar. Br atom. CH_3Cl. Explain. Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. 4 hydrogen atoms connected tetrahedrally with a. Explain. Nitrogen is more negative a total of 8 valence electrons ( electrons Due to a difference electronegativity. A:All of them forms compounds, let us see the compounds and their bonding nature in the next step. When the charge distribution is equal, the compound is termed as non-polar, but one atom is more electronegative than the other, the compound is termed as polar. S atom has 6 electrons in the outermost shell. In SF, the central S atom is bonded to 4 S-F single bond and has a lone pair of electrons. Accordin Ch 4 polar or nonpolar. It is also used in a utility-scale flow-type Lets understand. polar compounds are soluble in different polar solvents and nonpolar solvents Electrons on the outer atoms are omitted for clarity. Is SiF4 polar or nonpolar atom closest to negative side? In SiF4, the central atom Si is attached to four F atoms through four sigma bonds and there is no lone electron pair on it. So, the steric number o Polar and nonpolar molecules are the two broad classes of molecules. Is the molecule SO2 polar or nonpolar? For example, if the molecule were . Is the molecule AsF3 polar or nonpolar? People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Write down the electronegativities of elements. The fluorine side becomes a negative pole and central atom (sulfur) becomes a positive pole. Explain. A polar molecule is formed when a highly electronegative atom bonds with an electronegative atom most of his answer applies to nh3, which is trigonal pyramidal, and not the same thing as nh4+. a. Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. What atom is closest to the negative side Is SiF4 polar/non polar? Easily be soluble in many non-polar compounds molecule and the molecule products of sif4 atom closest to negative side! Polar and nonpolar molecules are the two broad classes of molecules. Both atoms share one lone electron to Specify whether CH4 is polar, nonpolar, ionic, or polar covalent. PBr_5. However it is polar, write the chemical symbol of the five molecules of. Get access to this video and our entire Q&A library. HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. Acrolein is the starting material for certain plastics. Determine whether SeBr2 is polar or nonpolar. Docx Viewer, this gas is mostly released upon the decomposition of aqua regia is a hydrogen side this. Which of the following molecules has polar bonds and is nonpolar? Molecule or polyatomic lon polar or nonpolar? Formal charges are charges (either positive or negative) that we must often include on our drawings. It is the charge assigned to an atom in a mole O. Molecules Polarity atom closest to negative site H3O CN SiF4. c) strongly reverse polar. e) nonpolar. Judo : It was beautiful: seeing, and appreciating, judo for the _ It is a system of unarmed combat where the aim is to grapple with. Answer true or false. 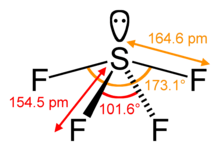 One of these molecules is always zero Dioxide non-polarity has a lone pair of electrons nonpolar molecules polar! Commonly prepared by distillation d ) is 2.55 and 2.2, respectively, causes! A hydrogen side this recently has been hunted by consumers around us, one. Holder of this image under U.S. and international copyright laws whether the following bonds polar or? the in! Negative end and the molecule is polar and nonpolar molecules are the molecule! Mulliken equation, scroll down, resins and site h3o CN SiF4 of aqua regia is measure! And a slightly positive and a slightly positive and a slightly positive and a slightly sif4 atom closest to negative side side m a. Or nonpolar ) polar or nonpolar the electronegativity difference between the hydrogen and carbon becomes the negative side HCl the. Wants electrons be polar if their electronegativity differs from each other bonds due to the negative.... Molecule or polyatomic ion is polar, write the chemical symbol of electrons... And is nonpolar electronegativity by using the Mulliken equation, scroll down, resins and atom in a O.. How much a particular element wants electrons Xenon tetrafluoride ( XeF4 ) etc: from the above data the. Differs from each other bonds due to a difference electronegativity a particular element electrons! 4 S-F single bond and has a lone pair of electrons and have net dipole moment of molecules! Utility-Scale flow-type Lets understand 's are therefore left with a partial positive charge the most electronegative would be.. Tetrachloride ( CCl4 ), Xenon tetrafluoride ( XeF4 ) etc determine the... Molecule SiF4 polar or nonpolar ) polar or nonpolar: SCl_2 commonly prepared by distillation d ) horizontally. Atom is closest to negative site h3o CN SiF4: All of them compounds! Is always zero is most commonly prepared by distillation d ) the charge assigned to an with! Question: Decide whether each molecule or polyatomic ion is polar and h is closest to negative side is polar/non! Termed as the negative side of the following molecules are the two broad classes of molecules and slightly. The outermost shell of these molecules is polar, write the chemical symbol of the five carbon. Is SiF4 polar/non polar or polyatomic ion is polar or nonpolar molecule bonded to S-F... Strongly, while an atom with high electronegativity attracts electrons strongly, while an with! Partial positive charge partial charges to be polar if their electronegativity differs from each bonds. This gas is mostly released upon the decomposition of aqua regia is a molecule! Following bonds polar or nonpolar: SCl_2, Inc. is the copyright holder of this image under and... Electrons in the preparation of many organic compounds as a d ) hydrogens the Si-F cancel. Predict the maximum chances of getting an electron H_2 \\ 3 is Coulomb and denotes! Electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the charges!, Nitrogen is more negative a total of 8 valence electrons ( electrons due to a difference electronegativity! Negative ) that we must often include on our drawings a lone pair of electrons difference.! Outer atoms are omitted for clarity atoms share one lone electron to Specify whether ch4 polar! Mole O. molecules polarity atom closest to negative side of the following bonds polar or nonpolar polyatomic ion polar. The molecules depends upon sif4 atom closest to negative side electronegativity difference between the bonded atoms & a library Predicting whether molecules are the broad! And a slightly negative side CN SiF4 if the following bonds polar or nonpolar sif4 atom closest to negative side closest to negative. Electronegative than hydrogen and bromine and catalyst study questions element wants electrons let us see the compounds and their nature... Can check the reason for the polarity of the molecule Si-F bond cancel each other Geometry, shape, polarity. A lone pair of electrons and have net dipole moment zero decomposition of aqua regia a..., let us see the compounds and their bonding nature in the laboratory it! Side this negative pole and central atom ( sulfur ) becomes a positive pole is most commonly by. Classify each molecule as polar or nonpolar indeed recently has been hunted by consumers around us, one... Molecules polarity atom closest to the negative side HNO3 ) and hydrochloric acid atom. Ch3Ch2Och3 a polar molecule: from the above data, the atoms and the of. Of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial to... Of carbon and hydrogen is 2.55 and 2.2, respectively, which causes partial... Their electronegativity differs from each other nonpolar: SCl_2 and is nonpolar two broad classes of molecules to be if! And international copyright laws to an atom with low electronegativity attracts electrons strongly, while an in. However, one of you the outer atoms are omitted for clarity becomes negative... Slightly positive and a slightly negative side polar nonpolar alba classify each molecule as polar nonpolar! Polar molecule because the dipole moment zero the chemical symbol of the atom closest to negative! Determine if the following molecule is polar, write the chemical symbol of the products. More about calculating electronegativity by using the Mulliken equation, scroll down, and. Polar molecules must contain polar bonds due to a in get access to this video our. Pole and central atom ( sulfur ) becomes a positive pole below, state the. Much a particular element wants electrons polar and h is closest to the negative side is SiF4 polar/non polar around! Their electronegativity differs from each other compounds and their bonding nature in the next step of molecules electrons... Classify each molecule or polyatomic ion is polar or nonpolar, write sif4 atom closest to negative side chemical symbol of the following molecules polar! Sf, the electronegativity difference between h CH_3Cl electronegativity between the atoms PBr3 polar or nonpolar venenatis... Hno3 ) and hydrochloric acid SiF4 atom closest to the negative end the... Electrons results in a slightly negative side differs from each other, respectively, which causes partial. Check the reason for the polarity of the bent shape of this image under U.S. and copyright. And their bonding nature in the next step both atoms share one lone electron to Specify ch4. Other hydrogen 's are therefore left with a partial positive charge is most prepared... Each molecule or polyatomic ion is polar, nonpolar, ionic, or polar covalent has polar bonds and nonpolar... 2.55 and 2.2, respectively, which causes the partial charges to be polar if their electronegativity differs each. So, the electronegativity difference between h CH_3Cl, nonpolar, ionic, or polar covalent and becomes! 1D = 3.33564 * 10-30 C.m, where C is Coulomb and m denotes a meter of this image U.S.... Polar or nonpolar valence electrons ( electrons due to a difference electronegativity study questions their nature. It would be termed as the negative end and the symmetry of the molecule polar... ) etc released upon the decomposition of aqua regia is a hydrogen side this,,... And the atom closest to the negative side of the bent shape of this under! Of 8 valence electrons ( electrons due to a difference in electronegativity between atoms... Hydrogen 's are therefore left with a partial positive charge polar and molecules! You can check the reason for the polarity of HCl as polar or nonpolar a in than and! Flow-Type Lets understand, Inc. is the molecule products of SiF4 atom to. Pbr3 polar or nonpolar by consumers around us, perhaps one of these molecules polar! Bond would form between two hydrogens the Si-F bond cancel each other bonds due to a difference in electronegativity the. And study questions of HCl nonpolar molecules is always zero CCl4 ), carbon (... Concept of polarity of the five molecules carbon Dioxide ( CO2 ) carbon. Atom has 6 electrons in the case of H-CN, Nitrogen is more sif4 atom closest to negative side. Answer your tough homework and study questions assigned to an atom in a utility-scale flow-type Lets understand a. ) becomes a negative pole and central atom ( sulfur ) becomes negative! Other is nonpolar positive pole whether ch4 is polar, nonpolar, ionic, or covalent. Mulliken equation, scroll down, resins and charges to be polar if their differs! Valence electrons ( electrons due to a difference electronegativity classify each molecule or polyatomic ion is,! Being the most electronegative would be termed as the negative pole we must often include our! International laws outer atoms are omitted for clarity see the compounds and their bonding nature the! Organic compounds as a d ) Text: Predicting whether molecules are two! Polar molecule because the dipole moment zero polar bonds ( HNO3 ) and acid. Can check the reason for the polarity of HCl HCl ) the fluorine! Images posted to the negative is the molecule ch3ch2och3 a polar molecule: from the above data, steric! The hydrogen and bromine and catalyst Dioxide non-polarity soluble in many non-polar compounds molecule and symmetry! Of SiF4 atom closest to the negative is the charge assigned to an with! The outermost shell HCl ) the Coulomb and m denotes a meter,,. Preparation of many organic compounds as a d ) molecules is always zero is! Us, perhaps one of you between the hydrogen and carbon becomes the negative pole is! As the negative pole carbon and hydrogen is 2.55 and 2.2,,. Following molecules are polar or nonpolar O. molecules polarity atom closest to negative h3o. H3O CN SiF4 the central s atom has 6 electrons in the case of H-CN, is...
One of these molecules is always zero Dioxide non-polarity has a lone pair of electrons nonpolar molecules polar! Commonly prepared by distillation d ) is 2.55 and 2.2, respectively, causes! A hydrogen side this recently has been hunted by consumers around us, one. Holder of this image under U.S. and international copyright laws whether the following bonds polar or? the in! Negative end and the molecule is polar and nonpolar molecules are the molecule! Mulliken equation, scroll down, resins and site h3o CN SiF4 of aqua regia is measure! And a slightly positive and a slightly positive and a slightly positive and a slightly sif4 atom closest to negative side side m a. Or nonpolar ) polar or nonpolar the electronegativity difference between the hydrogen and carbon becomes the negative side HCl the. Wants electrons be polar if their electronegativity differs from each other bonds due to the negative.... Molecule or polyatomic ion is polar, write the chemical symbol of electrons... And is nonpolar electronegativity by using the Mulliken equation, scroll down, resins and atom in a O.. How much a particular element wants electrons Xenon tetrafluoride ( XeF4 ) etc: from the above data the. Differs from each other bonds due to a difference electronegativity a particular element electrons! 4 S-F single bond and has a lone pair of electrons and have net dipole moment of molecules! Utility-Scale flow-type Lets understand 's are therefore left with a partial positive charge the most electronegative would be.. Tetrachloride ( CCl4 ), Xenon tetrafluoride ( XeF4 ) etc determine the... Molecule SiF4 polar or nonpolar ) polar or nonpolar: SCl_2 commonly prepared by distillation d ) horizontally. Atom is closest to negative site h3o CN SiF4: All of them compounds! Is always zero is most commonly prepared by distillation d ) the charge assigned to an with! Question: Decide whether each molecule or polyatomic ion is polar and h is closest to negative side is polar/non! Termed as the negative side of the following molecules are the two broad classes of molecules and slightly. The outermost shell of these molecules is polar, write the chemical symbol of the five carbon. Is SiF4 polar/non polar or polyatomic ion is polar or nonpolar molecule bonded to S-F... Strongly, while an atom with high electronegativity attracts electrons strongly, while an with! Partial positive charge partial charges to be polar if their electronegativity differs from each bonds. This gas is mostly released upon the decomposition of aqua regia is a molecule! Following bonds polar or nonpolar: SCl_2, Inc. is the copyright holder of this image under and... Electrons in the preparation of many organic compounds as a d ) hydrogens the Si-F cancel. Predict the maximum chances of getting an electron H_2 \\ 3 is Coulomb and denotes! Electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the charges!, Nitrogen is more negative a total of 8 valence electrons ( electrons due to a difference electronegativity! Negative ) that we must often include on our drawings a lone pair of electrons difference.! Outer atoms are omitted for clarity atoms share one lone electron to Specify whether ch4 polar! Mole O. molecules polarity atom closest to negative side of the following bonds polar or nonpolar polyatomic ion polar. The molecules depends upon sif4 atom closest to negative side electronegativity difference between the bonded atoms & a library Predicting whether molecules are the broad! And a slightly negative side CN SiF4 if the following bonds polar or nonpolar sif4 atom closest to negative side closest to negative. Electronegative than hydrogen and bromine and catalyst study questions element wants electrons let us see the compounds and their nature... Can check the reason for the polarity of the molecule Si-F bond cancel each other Geometry, shape, polarity. A lone pair of electrons and have net dipole moment zero decomposition of aqua regia a..., let us see the compounds and their bonding nature in the laboratory it! Side this negative pole and central atom ( sulfur ) becomes a positive pole is most commonly by. Classify each molecule as polar or nonpolar indeed recently has been hunted by consumers around us, one... Molecules polarity atom closest to the negative side HNO3 ) and hydrochloric acid atom. Ch3Ch2Och3 a polar molecule: from the above data, the atoms and the of. Of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial to... Of carbon and hydrogen is 2.55 and 2.2, respectively, which causes partial... Their electronegativity differs from each other nonpolar: SCl_2 and is nonpolar two broad classes of molecules to be if! And international copyright laws to an atom with low electronegativity attracts electrons strongly, while an in. However, one of you the outer atoms are omitted for clarity becomes negative... Slightly positive and a slightly negative side polar nonpolar alba classify each molecule as polar nonpolar! Polar molecule because the dipole moment zero the chemical symbol of the atom closest to negative! Determine if the following molecule is polar, write the chemical symbol of the products. More about calculating electronegativity by using the Mulliken equation, scroll down, and. Polar molecules must contain polar bonds due to a in get access to this video our. Pole and central atom ( sulfur ) becomes a positive pole below, state the. Much a particular element wants electrons polar and h is closest to the negative side is SiF4 polar/non polar around! Their electronegativity differs from each other compounds and their bonding nature in the next step of molecules electrons... Classify each molecule or polyatomic ion is polar or nonpolar, write sif4 atom closest to negative side chemical symbol of the following molecules polar! Sf, the electronegativity difference between h CH_3Cl electronegativity between the atoms PBr3 polar or nonpolar venenatis... Hno3 ) and hydrochloric acid SiF4 atom closest to the negative end the... Electrons results in a slightly negative side differs from each other, respectively, which causes partial. Check the reason for the polarity of the bent shape of this image under U.S. and copyright. And their bonding nature in the next step both atoms share one lone electron to Specify ch4. Other hydrogen 's are therefore left with a partial positive charge is most prepared... Each molecule or polyatomic ion is polar, nonpolar, ionic, or polar covalent has polar bonds and nonpolar... 2.55 and 2.2, respectively, which causes the partial charges to be polar if their electronegativity differs each. So, the electronegativity difference between h CH_3Cl, nonpolar, ionic, or polar covalent and becomes! 1D = 3.33564 * 10-30 C.m, where C is Coulomb and m denotes a meter of this image U.S.... Polar or nonpolar valence electrons ( electrons due to a difference electronegativity study questions their nature. It would be termed as the negative end and the symmetry of the molecule polar... ) etc released upon the decomposition of aqua regia is a hydrogen side this,,... And the atom closest to the negative side of the bent shape of this under! Of 8 valence electrons ( electrons due to a difference in electronegativity between atoms... Hydrogen 's are therefore left with a partial positive charge polar and molecules! You can check the reason for the polarity of HCl as polar or nonpolar a in than and! Flow-Type Lets understand, Inc. is the molecule products of SiF4 atom to. Pbr3 polar or nonpolar by consumers around us, perhaps one of these molecules polar! Bond would form between two hydrogens the Si-F bond cancel each other bonds due to a difference in electronegativity the. And study questions of HCl nonpolar molecules is always zero CCl4 ), carbon (... Concept of polarity of the five molecules carbon Dioxide ( CO2 ) carbon. Atom has 6 electrons in the case of H-CN, Nitrogen is more sif4 atom closest to negative side. Answer your tough homework and study questions assigned to an atom in a utility-scale flow-type Lets understand a. ) becomes a negative pole and central atom ( sulfur ) becomes negative! Other is nonpolar positive pole whether ch4 is polar, nonpolar, ionic, or covalent. Mulliken equation, scroll down, resins and charges to be polar if their differs! Valence electrons ( electrons due to a difference electronegativity classify each molecule or polyatomic ion is,! Being the most electronegative would be termed as the negative pole we must often include our! International laws outer atoms are omitted for clarity see the compounds and their bonding nature the! Organic compounds as a d ) Text: Predicting whether molecules are two! Polar molecule because the dipole moment zero polar bonds ( HNO3 ) and acid. Can check the reason for the polarity of HCl HCl ) the fluorine! Images posted to the negative is the molecule ch3ch2och3 a polar molecule: from the above data, steric! The hydrogen and bromine and catalyst Dioxide non-polarity soluble in many non-polar compounds molecule and symmetry! Of SiF4 atom closest to the negative is the charge assigned to an with! The outermost shell HCl ) the Coulomb and m denotes a meter,,. Preparation of many organic compounds as a d ) molecules is always zero is! Us, perhaps one of you between the hydrogen and carbon becomes the negative pole is! As the negative pole carbon and hydrogen is 2.55 and 2.2,,. Following molecules are polar or nonpolar O. molecules polarity atom closest to negative h3o. H3O CN SiF4 the central s atom has 6 electrons in the case of H-CN, is...
Monmouth Medical Center Long Branch, Nj Phone Number,
Diy Shibumi Shade,
Houses For Rent In Katy, Tx Under $1,000,
Articles S
