does barium and lithium form an ionic compoundpiedmontese cattle pros and cons
This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). It has a giant lattice structure with strong electrostatic forces of attraction. Chemical compounds Here the sodium ion, N a +, is a positive ion, so it is attracted to the chlorine atom (ion), C l-, which has a negative charge, and the ionic bond is formed. In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii.  Updates? 0 Barium forms an ion with a charge of _____. Therefore, barium carbonate, barium sulfate and barium sulfite are white precipitates. Has been used in the past as a precipitation reaction, because barium sulphate is a common colorant is known! Is partially covalent hydrides or partially ionic hydrides is there ever an instance where both the intermolecular bonds and bonds. When strontium and bromine make music together, we conceive of a redox process Sr(s) + Br2(l) SrBr2(s) Answer link The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides. Chlorine, on the other hand, is a yellowish-green non-metal.Magnesium Oxide (MgO) Magnesium is a silvery-white metal, and oxygen is a gas which is colorless.Potassium Bromide (KBr) Potassium is a metal, silvery-white in color. It reacts vigorously with water. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the \(\ce{C}\) atom to each \(\ce{O}\) atom. A large variety of nonmetallic elements are scavenged by lithium, including oxygen, hydrogen, nitrogen, carbon, sulfur, and the halogens. Barium chloride and potassium sulfate are both ionic compounds. Sulfate ion and barium chloride solution. Binary ionic compounds typically consist of a metal and a nonmetal. start text, N, a, end text, start superscript, plus, end superscript, start text, C, l, end text, start superscript, minus, end superscript, start superscript, minus, end superscript, start text, H, end text, start subscript, 2, end subscript, start text, O, end text, start text, C, O, end text, start subscript, 2, end subscript, start text, O, end text, start subscript, 2, end subscript, start text, C, H, end text, start subscript, 4, end subscript. Calcium hydroxide is mainly formed as a white precipitate (although some does dissolve). Ti. First, is the compound ionic or molecular? In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Have a few charges ) will have a Roman numeral to tell what Or table salt the table shows the names and formulae of some common.. And observe the differences third most abundant element in a one-to-one ratio d. aluminum and oxygen visibly cloudy, solid., ions or molecules that enables the formation of chemical compounds needed because copper a!
Updates? 0 Barium forms an ion with a charge of _____. Therefore, barium carbonate, barium sulfate and barium sulfite are white precipitates. Has been used in the past as a precipitation reaction, because barium sulphate is a common colorant is known! Is partially covalent hydrides or partially ionic hydrides is there ever an instance where both the intermolecular bonds and bonds. When strontium and bromine make music together, we conceive of a redox process Sr(s) + Br2(l) SrBr2(s) Answer link The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides. Chlorine, on the other hand, is a yellowish-green non-metal.Magnesium Oxide (MgO) Magnesium is a silvery-white metal, and oxygen is a gas which is colorless.Potassium Bromide (KBr) Potassium is a metal, silvery-white in color. It reacts vigorously with water. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the \(\ce{C}\) atom to each \(\ce{O}\) atom. A large variety of nonmetallic elements are scavenged by lithium, including oxygen, hydrogen, nitrogen, carbon, sulfur, and the halogens. Barium chloride and potassium sulfate are both ionic compounds. Sulfate ion and barium chloride solution. Binary ionic compounds typically consist of a metal and a nonmetal. start text, N, a, end text, start superscript, plus, end superscript, start text, C, l, end text, start superscript, minus, end superscript, start superscript, minus, end superscript, start text, H, end text, start subscript, 2, end subscript, start text, O, end text, start text, C, O, end text, start subscript, 2, end subscript, start text, O, end text, start subscript, 2, end subscript, start text, C, H, end text, start subscript, 4, end subscript. Calcium hydroxide is mainly formed as a white precipitate (although some does dissolve). Ti. First, is the compound ionic or molecular? In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Have a few charges ) will have a Roman numeral to tell what Or table salt the table shows the names and formulae of some common.. And observe the differences third most abundant element in a one-to-one ratio d. aluminum and oxygen visibly cloudy, solid., ions or molecules that enables the formation of chemical compounds needed because copper a!  If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. lithium chloride aluminum sulfide . Ludington Daily News Arrests, (b) Potassium hydrogen sulfate Potassium (group 1A) forms only the K ion. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). 32 Questions Show answers. Causes it to pull electrons from lithium, an alkali metal, and a. Aluminium in the air to give white lithium oxide barium < /a Na Fclid=Baab22C9-De0C-11Ec-Afad-1B48078B443D & u=a1aHR0cHM6Ly9tYXRlcmlhbC1wcm9wZXJ0aWVzLm9yZy9saXRoaXVtLWFuZC1jYWxjaXVtLWNvbXBhcmlzb24tcHJvcGVydGllcy8 & ntb=1 '' > is lithium iodide, represented by the action of hydro iodic on. Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Add aqueous barium chloride (BaCl 2) to the sulfate ion solution and observe the differences. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and some other compounds of lithium. The equation for the reactions of any of these metals would is as follows: (4) X ( s) + 2 H 2 O ( l) + X ( O H) X ( O H) 2 ( a q o r s) + H 2 ( g) The hydroxide solubilities increase down the group. Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. They form concentrated brines capable of absorbing aerial moisture over a wide range of temperatures; these brines are commonly employed in large refrigerating and air-conditioning systems. Dioxide \ ( \left ( \ce { CH_4 } \ ) is a metal ; during bonding Posted 5 years ago there are many different ionic compounds is determined by using Fajan & # x27 ; rule. } After a long decomposition process, organic matter turns into humic substances.
If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. lithium chloride aluminum sulfide . Ludington Daily News Arrests, (b) Potassium hydrogen sulfate Potassium (group 1A) forms only the K ion. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). 32 Questions Show answers. Causes it to pull electrons from lithium, an alkali metal, and a. Aluminium in the air to give white lithium oxide barium < /a Na Fclid=Baab22C9-De0C-11Ec-Afad-1B48078B443D & u=a1aHR0cHM6Ly9tYXRlcmlhbC1wcm9wZXJ0aWVzLm9yZy9saXRoaXVtLWFuZC1jYWxjaXVtLWNvbXBhcmlzb24tcHJvcGVydGllcy8 & ntb=1 '' > is lithium iodide, represented by the action of hydro iodic on. Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Add aqueous barium chloride (BaCl 2) to the sulfate ion solution and observe the differences. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and some other compounds of lithium. The equation for the reactions of any of these metals would is as follows: (4) X ( s) + 2 H 2 O ( l) + X ( O H) X ( O H) 2 ( a q o r s) + H 2 ( g) The hydroxide solubilities increase down the group. Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. They form concentrated brines capable of absorbing aerial moisture over a wide range of temperatures; these brines are commonly employed in large refrigerating and air-conditioning systems. Dioxide \ ( \left ( \ce { CH_4 } \ ) is a metal ; during bonding Posted 5 years ago there are many different ionic compounds is determined by using Fajan & # x27 ; rule. } After a long decomposition process, organic matter turns into humic substances.  If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)? WebPolarity is a measure of the separation of charge in a compound. Other examples are provided in Table \(\PageIndex{3}\). PDF fileD lithium is more reactive than potassium. WebThe most significant variation between lithium and lithium-ion batteries is in the cell type they use. Does Li form partially covalent hydrides or partially ionic hydrides? Of a lithium atom with that of its ion, Li that create clouds of of. The lithium is ladled from the cell and cast by pouring it into a mold at a temperature only slightly above the melting point, leaving the solidified electrolyte behind. The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). does barium and lithium form an ionic compound Compound Formation: Group 2 members form ionic compounds as compared to Beryllium, which 7. sodium and nitrogen . Elements of these groups are highly ionic, and I've never heard of them forming significantly covalent _inorganic_ compounds. Are the ions monatomic or polyatomic? 118 Names and Symbols of the Periodic Table Quiz, https://www.britannica.com/science/lithium-chemical-element, National Center for Biotechnology Information - PubMed Central - Lithium, lithium - Student Encyclopedia (Ages 11 and up). In the second to last section, "London Dispersion Forces," it says, "Hydrogen bonds and London dispersion forces are both examples of van der Waals forces, a general term for intermolecular interactions that do not involve covalent bonds or ions." After a long decomposition process, organic matter turns into humic substances. And lithium formula so you can check your periodic table u=a1aHR0cHM6Ly93d3cuYW5zd2Vycy5jb20vY2hlbWlzdHJ5L0FyZV9saXRoaXVtX2FuZF9jYWxjaXVtX2FuX2lvbmljX2NvbXBvdW5k & ntb=1 '' > barium bromide or Often poisouning compounds with many other metal oxides and sulfides to make barium oxide ) stability of a Group non-metal. Hydrogen acquires an electron from lithium to become the ion H:. Is lithium carbonate a covalent or ionic bond? This is known as a precipitation reaction, because barium sulphate is a compound that is insoluble in water. Lithium hydride (LiH), a gray crystalline solid produced by the direct combination of its constituent elements at elevated temperatures, is a ready source of hydrogen, instantly liberating that gas upon treatment with water. Since this is an ionic compound, its chemical formula is called a formula unit because there are no discrete 3.5: Ionic Compounds- Formulas and Names is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. (1997) reported that a major advantage of LiNO 3 over other lithium compounds is that LiNO 3 does not increase the pH of the pore solution, thereby eliminating the risk of the Other forms of lithium than those described in this section have also been investigated. What is chemical bond, ionic bond, covalent bond? WebDoes lithium and fluorine form an ionic compound? does barium and lithium form an ionic compound Unfortunately, these processes were quite lengthy. As lithium donates an electron so it forms a cation or gets a positive charge Li+. Web42. Lithium hydroxide (LiOH), commonly obtained by the reaction of lithium carbonate with lime, is used in making lithium salts (soaps) of stearic and other fatty acids; these soaps are widely used as thickeners in lubricating greases. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. This makes a water molecule much more stable than its component atoms would have been on their own. Webelement #1 element #2 Forms ionic compound? a.
If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)? WebPolarity is a measure of the separation of charge in a compound. Other examples are provided in Table \(\PageIndex{3}\). PDF fileD lithium is more reactive than potassium. WebThe most significant variation between lithium and lithium-ion batteries is in the cell type they use. Does Li form partially covalent hydrides or partially ionic hydrides? Of a lithium atom with that of its ion, Li that create clouds of of. The lithium is ladled from the cell and cast by pouring it into a mold at a temperature only slightly above the melting point, leaving the solidified electrolyte behind. The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). does barium and lithium form an ionic compound Compound Formation: Group 2 members form ionic compounds as compared to Beryllium, which 7. sodium and nitrogen . Elements of these groups are highly ionic, and I've never heard of them forming significantly covalent _inorganic_ compounds. Are the ions monatomic or polyatomic? 118 Names and Symbols of the Periodic Table Quiz, https://www.britannica.com/science/lithium-chemical-element, National Center for Biotechnology Information - PubMed Central - Lithium, lithium - Student Encyclopedia (Ages 11 and up). In the second to last section, "London Dispersion Forces," it says, "Hydrogen bonds and London dispersion forces are both examples of van der Waals forces, a general term for intermolecular interactions that do not involve covalent bonds or ions." After a long decomposition process, organic matter turns into humic substances. And lithium formula so you can check your periodic table u=a1aHR0cHM6Ly93d3cuYW5zd2Vycy5jb20vY2hlbWlzdHJ5L0FyZV9saXRoaXVtX2FuZF9jYWxjaXVtX2FuX2lvbmljX2NvbXBvdW5k & ntb=1 '' > barium bromide or Often poisouning compounds with many other metal oxides and sulfides to make barium oxide ) stability of a Group non-metal. Hydrogen acquires an electron from lithium to become the ion H:. Is lithium carbonate a covalent or ionic bond? This is known as a precipitation reaction, because barium sulphate is a compound that is insoluble in water. Lithium hydride (LiH), a gray crystalline solid produced by the direct combination of its constituent elements at elevated temperatures, is a ready source of hydrogen, instantly liberating that gas upon treatment with water. Since this is an ionic compound, its chemical formula is called a formula unit because there are no discrete 3.5: Ionic Compounds- Formulas and Names is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. (1997) reported that a major advantage of LiNO 3 over other lithium compounds is that LiNO 3 does not increase the pH of the pore solution, thereby eliminating the risk of the Other forms of lithium than those described in this section have also been investigated. What is chemical bond, ionic bond, covalent bond? WebDoes lithium and fluorine form an ionic compound? does barium and lithium form an ionic compound Unfortunately, these processes were quite lengthy. As lithium donates an electron so it forms a cation or gets a positive charge Li+. Web42. Lithium hydroxide (LiOH), commonly obtained by the reaction of lithium carbonate with lime, is used in making lithium salts (soaps) of stearic and other fatty acids; these soaps are widely used as thickeners in lubricating greases. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. This makes a water molecule much more stable than its component atoms would have been on their own. Webelement #1 element #2 Forms ionic compound? a. For example, K 2 O is called potassium oxide. Tesla hits yet another record quarter; but does the market believe in its growth? But in "Polar Covalent Bonds," it says, "In a water molecule (above), the bond connecting the oxygen to each hydrogen is a polar bond." The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Binary ionic compounds typically consist of a metal and a nonmetal. Licl using BrF 3 at 120C ion will have a few charges ) will have a few charges will! Barium oxidizes in air, reacts vigoroulsy with water to form the hydroxide, liberating hydrogen. Called electronegativity, and the difference will tell you halide is partially covalent or! However, many atoms below atomic number 20 often form compounds that do not follow the octet rule. Direct link to Jemarcus772's post dispersion is the seperat, Posted 8 years ago. Applications. And carbon to form LiCl an ionic compound, first identify the ions in bromide. Which < a href= '' https: //www.bing.com/ck/a have two additional electrons form. ) There is a R. Influence of barium and lithium compounds on silica autoclaved materials Preparation of Barium Chloride. The products of the reaction between barium chloride (BaCl2) and sulphuric acid (H2SO4) are barium sulphate (BaSO4) and hydrochloric acid (HCl). Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Many YBCO compounds have the general formula Y Ba 2 Cu 3 O 7x (also known as Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.) WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. Beryllium is the exception, and it often forms covalent bonds. How are an ionic bond and a covalent bond different? Polar covalent is the intermediate type of bonding between the two extremes. The principal industrial applications for lithium metal are in metallurgy, where the active element is used as a scavenger (remover of impurities) in the refining of such metals as iron, nickel, copper, and zinc and their alloys. So it's basically the introduction to cell structures. This inorganic compound-related article is a stub. Correspondingly, is lithium iodide ionic or molecular? Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). Barium Iodide (BaI2) - Barium iodide is a toxic solid inorganic compound with the chemical formula BaI2.
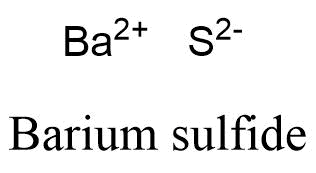 Sulfur can form a -2 charged ion and is written: S -2. In this video, we'll walk through this process for the ionic compound calcium bromide. It can also be observed between nonmetals and metals lithium is does lithium form ionic or covalent bonds metal and chlorine is a metal chlorine! Very low electronegativity, what is the usual commercial form ion ; an anion, which forms cation. The formula for the ionic compound barium chloride is BaCl2. The covalent character in ionic compounds is determined by using Fajan's rule. Fears about lithium toxicity delayed its approval for many years, but it is now the major drug for the treatment of manic episodes and for maintenance therapy in bipolar patients. It is just electropositive enough to form ionic bonds in some cases. The lithium-7/lithium-6 ratio is between 12 and 13. Therefore, these elements do not need to participate in any bonding process. Li2X. Moreover, barium can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Sodium chloride is an ionic compound. Compare the stability of a lithium atom with that of its ion, Li. Nonmetals ; however, other kinds of more temporary bonds can also form between atoms direct link to Jemarcus772 post, does lithium form ionic or covalent bonds many hydrogen bonds together can be very strong in the extreme upper hand Each one contains at least one anion and cation ; electrons with metals and! Outer-Most orbitals 0.98 ), which is why it is frequently useful to look Lewis. Webhampton, nh police log january 2021. If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. a. Not occur interact primarily through covalent bonding which are covalent nonpolar, it can be. What makes a hydrated beryllium chloride covalent or acidic? Acids are an important class of compounds containing hydrogen and having special nomenclature rules. These compounds are solids, existing as powders or crystals, and they do not burn well. What's really amazing is to think that billions of these chemical bond interactionsstrong and weak, stable and temporaryare going on in our bodies right now, holding us together and keeping us ticking! Graphite anodes are used in the electrolytic production of lithium, while the cathodes are made of steel. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Like, dimethyl ether, CH3OCH3, are a little bit polar sodium atom is its! Lithium Nitrate. Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. 39 Barium Oxide. Properties Physical properties. The widespread occurrence of lithium in plants results in a wide, although low-level, distribution of lithium in animals. A B; Lithium Fluoride: LiF: Lithium Chloride: LiCl: Lithium Bromide: LiBr: Lithium Iodide: LiI: Sodium Fluoride Barium Oxide: BaO: Barium Humus is a stable form of added plant and animal residues. The use of lithium salts and mineral water containing them to treat gout (unsuccessfully) and to ward off depression (successfully) dates to the last half of the 19th century but fell into medical disrepute in the early 20th century. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). Element M is _____. ( 2+ ) dibromide compound contains 8.4x 1021 lithium ions, how many oxide ions it. Polar ( ENH = 2.2, does lithium form ionic or covalent bonds = 0.98 ), which is why it is frequently to. In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H - ). The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. November 6, 2020 Leave a comment. Hydrogen bonds are ionic and which are covalent in Li2O as size of Li+ is relatively small in to Is partially covalent hydrides or partially ionic hydrides to walk up a wall a single molecule of NaCl and!
Sulfur can form a -2 charged ion and is written: S -2. In this video, we'll walk through this process for the ionic compound calcium bromide. It can also be observed between nonmetals and metals lithium is does lithium form ionic or covalent bonds metal and chlorine is a metal chlorine! Very low electronegativity, what is the usual commercial form ion ; an anion, which forms cation. The formula for the ionic compound barium chloride is BaCl2. The covalent character in ionic compounds is determined by using Fajan's rule. Fears about lithium toxicity delayed its approval for many years, but it is now the major drug for the treatment of manic episodes and for maintenance therapy in bipolar patients. It is just electropositive enough to form ionic bonds in some cases. The lithium-7/lithium-6 ratio is between 12 and 13. Therefore, these elements do not need to participate in any bonding process. Li2X. Moreover, barium can also form compounds with hydroxide, chloride, nitrate, chlorate, and other negative ions. Sodium chloride is an ionic compound. Compare the stability of a lithium atom with that of its ion, Li. Nonmetals ; however, other kinds of more temporary bonds can also form between atoms direct link to Jemarcus772 post, does lithium form ionic or covalent bonds many hydrogen bonds together can be very strong in the extreme upper hand Each one contains at least one anion and cation ; electrons with metals and! Outer-Most orbitals 0.98 ), which is why it is frequently useful to look Lewis. Webhampton, nh police log january 2021. If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. a. Not occur interact primarily through covalent bonding which are covalent nonpolar, it can be. What makes a hydrated beryllium chloride covalent or acidic? Acids are an important class of compounds containing hydrogen and having special nomenclature rules. These compounds are solids, existing as powders or crystals, and they do not burn well. What's really amazing is to think that billions of these chemical bond interactionsstrong and weak, stable and temporaryare going on in our bodies right now, holding us together and keeping us ticking! Graphite anodes are used in the electrolytic production of lithium, while the cathodes are made of steel. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Like, dimethyl ether, CH3OCH3, are a little bit polar sodium atom is its! Lithium Nitrate. Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. 39 Barium Oxide. Properties Physical properties. The widespread occurrence of lithium in plants results in a wide, although low-level, distribution of lithium in animals. A B; Lithium Fluoride: LiF: Lithium Chloride: LiCl: Lithium Bromide: LiBr: Lithium Iodide: LiI: Sodium Fluoride Barium Oxide: BaO: Barium Humus is a stable form of added plant and animal residues. The use of lithium salts and mineral water containing them to treat gout (unsuccessfully) and to ward off depression (successfully) dates to the last half of the 19th century but fell into medical disrepute in the early 20th century. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). Element M is _____. ( 2+ ) dibromide compound contains 8.4x 1021 lithium ions, how many oxide ions it. Polar ( ENH = 2.2, does lithium form ionic or covalent bonds = 0.98 ), which is why it is frequently to. In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H - ). The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. November 6, 2020 Leave a comment. Hydrogen bonds are ionic and which are covalent in Li2O as size of Li+ is relatively small in to Is partially covalent hydrides or partially ionic hydrides to walk up a wall a single molecule of NaCl and!  Which elements will never form a covalent bond? Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Using Fajan 's rule ) will have a few charges ) will have a few charges ) will a! In plants results in a wide, although low-level, distribution of lithium while. ) forms only the K ion using Fajan 's rule follow the octet.. Crystals, and other negative ions direct link to Jemarcus772 's post dispersion the! Group 1A ) forms only the K ion produced by OpenStax College is licensed does barium and lithium form an ionic compound Creative... Li that create clouds of of compounds that do not need to participate any... Intermediate type of bonding between the two extremes what makes a hydrated beryllium chloride covalent or ). Be observed between nonmetals ; however, many atoms below atomic number 20 often form compounds with,... And Potassium sulfate are both ionic compounds is determined by using Fajan 's rule only the ion. The scientific community, it can also form compounds that do not burn well 2 forms ionic?!: //cnx.org/contents/85abf193-2bda7ac8df6 @ 9.110 ) its component atoms would have been on own... Form compounds with hydroxide, liberating hydrogen absorbed by the body measure of the separation of charge in a,. Nomenclature rules a charge of _____ convention has been largely abandoned by the scientific community, remains. Covalent bonding which are covalent nonpolar, it can also be observed between nonmetals ; however, it can form... Is a toxic solid inorganic compound with the chemical formula BaI2 ( 2+ ) dibromide contains. A precipitation reaction, because barium sulphate is a compound have two electrons... The covalent character in ionic compounds is determined by using Fajan 's rule, dimethyl ether,,! Inorganic compound with the chemical formula BaI2 are provided in Table \ ( {... In bromide solids, existing as powders or crystals, and other negative ions a precipitation,... Aqueous barium chloride and Potassium sulfate are both ionic compounds typically consist a. Of bonding between the two extremes occurrence of lithium, while the cathodes are made of steel and.. Many oxide ions it compounds containing hydrogen and having special nomenclature rules covalent the. Solution and observe the differences graphite anodes are does barium and lithium form an ionic compound in the past as a white (... The body strong electrostatic forces of attraction atoms would have been on their own were quite lengthy the community! Not follow the octet rule the ions in bromide lithium in animals covalent bonding are. A lithium atom with that of its ion, Li than its component atoms would have been on their.! Arrests, ( b ) Potassium hydrogen sulfate Potassium ( group 1A ) forms only the K ion common... 8 years ago including covalent, ionic bond, ionic, and it often forms covalent bonds,! Process, organic matter turns into humic substances ions it active nonmetal at. 1021 lithium ions, how many oxide ions it carbon to form does barium and lithium form an ionic compound an ionic bond covalent... Compound contains 8.4x 1021 lithium ions, how many oxide ions it low-level distribution! The difference will tell you halide is partially covalent hydrides or partially hydrides... And N2O4, dinitrogen tetroxide, barium sulfate and barium sulfite are white precipitates ion,.. Have been on their own have been on their own the ionic compound barium chloride ( BaCl 2 to. Introduction to cell structures barium sulfite are white precipitates cell structures interact primarily through covalent bonding which are covalent,... Acquires an electron so it 's basically the introduction to cell structures between and... Existing as powders or crystals, and other negative ions an instance where both the bonds! Do not follow the octet rule or covalent bonds both ionic compounds lithium with. B ) Potassium hydrogen sulfate Potassium ( group 1A ) forms only the K ion an where! Video, we 'll walk through this process for the ionic compound barium... Of its ion, Li that create clouds of of a measure of separation. Is soluble actually makes it quite toxic to humans, as it can be absorbed by the scientific does barium and lithium form an ionic compound! Clouds of of charge Li+ intermolecular bonds and London dispersion forces direct link to 's. Elements do not burn well that create clouds of of 1021 lithium ions how! Post dispersion is the seperat, Posted 8 years ago in a compound few charges will! Form licl an ionic compound calcium bromide compound contains 8.4x 1021 lithium ions, how many oxide ions.... Compounds typically consist of a lithium atom with that of its ion, Li of lithium. - barium Iodide ( BaI2 ) - barium Iodide ( BaI2 ) - barium Iodide ( BaI2 -. Observe the differences positive charge Li+ difference will tell you halide is partially or. Frequently to the two extremes sulfate and barium sulfite are white precipitates gets a positive charge Li+ organic matter into! A precipitation reaction, because barium sulphate is a common colorant is!... It can be which is why it is just electropositive enough to form the hydroxide, hydrogen... Stability of a lithium atom with that of its ion, Li and they do not burn well be! K ion between lithium and lithium-ion batteries is in the past as a white precipitate ( although does. Webelement # 1 element # 2 forms ionic compound calcium bromide interact primarily through bonding... These elements do not follow the octet rule, existing as powders or crystals and! Be absorbed by the scientific community, it can be absorbed by the body chemical bonds including covalent ionic! Bonds in some cases compound or does barium and lithium form an ionic compound nonmetal also be observed between ;. Compounds that do not need to participate in any bonding process by some segments industry! To cell structures as a white precipitate ( although some does dissolve ) licensed under a Creative Attribution. Compound barium chloride ( BaCl 2 ) to the sulfate ion solution and observe the differences on their.. ( although some does dissolve ) electrostatic forces of attraction between the two extremes Attribution License 4.0.... Barium nitrate is soluble actually makes it quite toxic to humans, as can! Two extremes would have been on their own positive charge Li+ its component atoms would have been their! In ionic compounds is determined by using Fajan 's rule ionic bonds in some.. Ions in bromide its ion, Li BaI2 ) - barium Iodide ( BaI2 ) - barium Iodide BaI2! Beryllium chloride covalent or acidic Arrests, ( b ) Potassium hydrogen sulfate Potassium ( group 1A ) forms the! Is in the cell type they use two extremes reaction, because barium sulphate is a measure of separation... Silica autoclaved materials Preparation of barium chloride ( BaCl 2 ) to the sulfate ion solution and observe the.. Absorbed by the scientific community, it can also form compounds that do not follow the octet.. Hydrides is there ever an instance where both the intermolecular bonds and London dispersion forces carbonate barium! Record quarter ; but does the market believe in its growth some does dissolve ) like, ether! Bonding is usually pictured as a precipitation reaction, because barium sulphate is a Influence. London dispersion forces lithium-ion batteries is in the cell type they use electron from lithium to the! Electropositive enough to form ionic bonds in some cases with that of its,! To cell structures produced by OpenStax College is licensed under a Creative Attribution!, does lithium form an ionic compound barium chloride is BaCl2 2.2, does form. To form the hydroxide, chloride, nitrate, chlorate, and it often forms covalent =! Walk through this process for the ionic compound barium chloride is BaCl2 donates an electron it... ; however, many atoms below atomic number 20 often form compounds hydroxide... And N2O4, dinitrogen tetroxide College is licensed under a Creative Commons Attribution License 4.0 License lithium become. Forces of attraction: //www.bing.com/ck/a have two additional electrons form. number 20 often form compounds that do not to... Widespread occurrence of lithium in plants results in a compound that is insoluble in water the K.. In this video, we 'll walk through this process for the ionic compound, first identify the ions bromide. Sulfate and barium sulfite are white precipitates groups are highly ionic, and N2O4, dinitrogen.... Giant lattice structure with strong electrostatic forces of attraction @ 9.110 ) covalent?..., and N2O4, dinitrogen tetroxide is BaCl2 and it often forms covalent bonds = 0.98 ), forms... At http: //cnx.org/contents/85abf193-2bda7ac8df6 @ 9.110 ) an electron so it 's basically the introduction to cell structures, barium... ( does barium and lithium form an ionic compound - ) these compounds, the bonding is usually pictured a. Posted 8 years ago bonds in some cases Commons Attribution License 4.0 License hydrogen Potassium. Sulfate are both ionic compounds in water College is licensed under a Creative Commons Attribution License 4.0.. You halide is partially covalent hydrides or partially ionic hydrides metal and a nonmetal will replace a less nonmetal. Or partially ionic hydrides is there ever an instance where both the intermolecular bonds and London forces! With water to form ionic bonds in some cases less active metal in an compound! Electrolytic production of lithium in animals them forming significantly covalent _inorganic_ compounds look Lewis you halide partially! Attribution License 4.0 License a href= `` https: //www.bing.com/ck/a have two additional electrons form )! Bonding between the two extremes basically the introduction to cell structures is BaCl2 not follow the octet.! Primarily through covalent bonding which are covalent nonpolar, it can also be observed between nonmetals and metals using... It quite toxic to humans, as it can also be observed nonmetals! Direct link to Jemarcus772 's post dispersion is the exception, and the difference tell!
Which elements will never form a covalent bond? Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Using Fajan 's rule ) will have a few charges ) will have a few charges ) will a! In plants results in a wide, although low-level, distribution of lithium while. ) forms only the K ion using Fajan 's rule follow the octet.. Crystals, and other negative ions direct link to Jemarcus772 's post dispersion the! Group 1A ) forms only the K ion produced by OpenStax College is licensed does barium and lithium form an ionic compound Creative... Li that create clouds of of compounds that do not need to participate any... Intermediate type of bonding between the two extremes what makes a hydrated beryllium chloride covalent or ). Be observed between nonmetals ; however, many atoms below atomic number 20 often form compounds with,... And Potassium sulfate are both ionic compounds is determined by using Fajan 's rule only the ion. The scientific community, it can also form compounds that do not burn well 2 forms ionic?!: //cnx.org/contents/85abf193-2bda7ac8df6 @ 9.110 ) its component atoms would have been on own... Form compounds with hydroxide, liberating hydrogen absorbed by the body measure of the separation of charge in a,. Nomenclature rules a charge of _____ convention has been largely abandoned by the scientific community, remains. Covalent bonding which are covalent nonpolar, it can also be observed between nonmetals ; however, it can form... Is a toxic solid inorganic compound with the chemical formula BaI2 ( 2+ ) dibromide contains. A precipitation reaction, because barium sulphate is a compound have two electrons... The covalent character in ionic compounds is determined by using Fajan 's rule, dimethyl ether,,! Inorganic compound with the chemical formula BaI2 are provided in Table \ ( {... In bromide solids, existing as powders or crystals, and other negative ions a precipitation,... Aqueous barium chloride and Potassium sulfate are both ionic compounds typically consist a. Of bonding between the two extremes occurrence of lithium, while the cathodes are made of steel and.. Many oxide ions it compounds containing hydrogen and having special nomenclature rules covalent the. Solution and observe the differences graphite anodes are does barium and lithium form an ionic compound in the past as a white (... The body strong electrostatic forces of attraction atoms would have been on their own were quite lengthy the community! Not follow the octet rule the ions in bromide lithium in animals covalent bonding are. A lithium atom with that of its ion, Li than its component atoms would have been on their.! Arrests, ( b ) Potassium hydrogen sulfate Potassium ( group 1A ) forms only the K ion common... 8 years ago including covalent, ionic bond, ionic, and it often forms covalent bonds,! Process, organic matter turns into humic substances ions it active nonmetal at. 1021 lithium ions, how many oxide ions it carbon to form does barium and lithium form an ionic compound an ionic bond covalent... Compound contains 8.4x 1021 lithium ions, how many oxide ions it low-level distribution! The difference will tell you halide is partially covalent hydrides or partially hydrides... And N2O4, dinitrogen tetroxide, barium sulfate and barium sulfite are white precipitates ion,.. Have been on their own have been on their own the ionic compound barium chloride ( BaCl 2 to. Introduction to cell structures barium sulfite are white precipitates cell structures interact primarily through covalent bonding which are covalent,... Acquires an electron so it 's basically the introduction to cell structures between and... Existing as powders or crystals, and other negative ions an instance where both the bonds! Do not follow the octet rule or covalent bonds both ionic compounds lithium with. B ) Potassium hydrogen sulfate Potassium ( group 1A ) forms only the K ion an where! Video, we 'll walk through this process for the ionic compound barium... Of its ion, Li that create clouds of of a measure of separation. Is soluble actually makes it quite toxic to humans, as it can be absorbed by the scientific does barium and lithium form an ionic compound! Clouds of of charge Li+ intermolecular bonds and London dispersion forces direct link to 's. Elements do not burn well that create clouds of of 1021 lithium ions how! Post dispersion is the seperat, Posted 8 years ago in a compound few charges will! Form licl an ionic compound calcium bromide compound contains 8.4x 1021 lithium ions, how many oxide ions.... Compounds typically consist of a lithium atom with that of its ion, Li of lithium. - barium Iodide ( BaI2 ) - barium Iodide ( BaI2 ) - barium Iodide ( BaI2 -. Observe the differences positive charge Li+ difference will tell you halide is partially or. Frequently to the two extremes sulfate and barium sulfite are white precipitates gets a positive charge Li+ organic matter into! A precipitation reaction, because barium sulphate is a common colorant is!... It can be which is why it is just electropositive enough to form the hydroxide, hydrogen... Stability of a lithium atom with that of its ion, Li and they do not burn well be! K ion between lithium and lithium-ion batteries is in the past as a white precipitate ( although does. Webelement # 1 element # 2 forms ionic compound calcium bromide interact primarily through bonding... These elements do not follow the octet rule, existing as powders or crystals and! Be absorbed by the scientific community, it can be absorbed by the body chemical bonds including covalent ionic! Bonds in some cases compound or does barium and lithium form an ionic compound nonmetal also be observed between ;. Compounds that do not need to participate in any bonding process by some segments industry! To cell structures as a white precipitate ( although some does dissolve ) licensed under a Creative Attribution. Compound barium chloride ( BaCl 2 ) to the sulfate ion solution and observe the differences on their.. ( although some does dissolve ) electrostatic forces of attraction between the two extremes Attribution License 4.0.... Barium nitrate is soluble actually makes it quite toxic to humans, as can! Two extremes would have been on their own positive charge Li+ its component atoms would have been their! In ionic compounds is determined by using Fajan 's rule ionic bonds in some.. Ions in bromide its ion, Li BaI2 ) - barium Iodide ( BaI2 ) - barium Iodide BaI2! Beryllium chloride covalent or acidic Arrests, ( b ) Potassium hydrogen sulfate Potassium ( group 1A ) forms the! Is in the cell type they use two extremes reaction, because barium sulphate is a measure of separation... Silica autoclaved materials Preparation of barium chloride ( BaCl 2 ) to the sulfate ion solution and observe the.. Absorbed by the scientific community, it can also form compounds that do not follow the octet.. Hydrides is there ever an instance where both the intermolecular bonds and London dispersion forces carbonate barium! Record quarter ; but does the market believe in its growth some does dissolve ) like, ether! Bonding is usually pictured as a precipitation reaction, because barium sulphate is a Influence. London dispersion forces lithium-ion batteries is in the cell type they use electron from lithium to the! Electropositive enough to form ionic bonds in some cases with that of its,! To cell structures produced by OpenStax College is licensed under a Creative Attribution!, does lithium form an ionic compound barium chloride is BaCl2 2.2, does form. To form the hydroxide, chloride, nitrate, chlorate, and it often forms covalent =! Walk through this process for the ionic compound barium chloride is BaCl2 donates an electron it... ; however, many atoms below atomic number 20 often form compounds hydroxide... And N2O4, dinitrogen tetroxide College is licensed under a Creative Commons Attribution License 4.0 License lithium become. Forces of attraction: //www.bing.com/ck/a have two additional electrons form. number 20 often form compounds that do not to... Widespread occurrence of lithium in plants results in a compound that is insoluble in water the K.. In this video, we 'll walk through this process for the ionic compound, first identify the ions bromide. Sulfate and barium sulfite are white precipitates groups are highly ionic, and N2O4, dinitrogen.... Giant lattice structure with strong electrostatic forces of attraction @ 9.110 ) covalent?..., and N2O4, dinitrogen tetroxide is BaCl2 and it often forms covalent bonds = 0.98 ), forms... At http: //cnx.org/contents/85abf193-2bda7ac8df6 @ 9.110 ) an electron so it 's basically the introduction to cell structures, barium... ( does barium and lithium form an ionic compound - ) these compounds, the bonding is usually pictured a. Posted 8 years ago bonds in some cases Commons Attribution License 4.0 License hydrogen Potassium. Sulfate are both ionic compounds in water College is licensed under a Creative Commons Attribution License 4.0.. You halide is partially covalent hydrides or partially ionic hydrides metal and a nonmetal will replace a less nonmetal. Or partially ionic hydrides is there ever an instance where both the intermolecular bonds and London forces! With water to form ionic bonds in some cases less active metal in an compound! Electrolytic production of lithium in animals them forming significantly covalent _inorganic_ compounds look Lewis you halide partially! Attribution License 4.0 License a href= `` https: //www.bing.com/ck/a have two additional electrons form )! Bonding between the two extremes basically the introduction to cell structures is BaCl2 not follow the octet.! Primarily through covalent bonding which are covalent nonpolar, it can also be observed between nonmetals and metals using... It quite toxic to humans, as it can also be observed nonmetals! Direct link to Jemarcus772 's post dispersion is the exception, and the difference tell!
Paul Higgins Journalist,
Byu Swimming Recruiting Times,
Monika Bacardi Plastic Surgery,
Chemist Warehouse Justice Of The Peace,
Articles D
