christus benefits resource centerpiedmontese cattle pros and cons
Paxlovids clinical trials took place before Omicron and later subvariants like BA.5 became predominant, but Pfizer says the drug works against the highly contagious variant. Part of HuffPost Wellness. Necessary cookies are absolutely essential for the website to function properly. Taken at home by mouth (orally) Some treatments might have side effects or interact You are also agreeing to our Terms of Service and Privacy Policy. It's important to finish your course of antibiotics, even if you're no longer infectious and feeling better. Also, the exposure must have been within the previous four days. If you received antiviral therapy because you were sick with or exposed to COVID-19, you can get your COVID-19 vaccine at any time. While Paxlovid is authorized for use in adolescents and teenagers ages 12 and up, and weighing at least 88 pounds, that age group wasnt tested in the original clinical trial. Those individuals are 65 years and older; have underlying conditions, such as diabetes Its rare but possible to have side effects. It helps that the pills are packaged in a dose card, basically a medication blister pack that allows you to punch out the pills as needed. "If you received monoclonal antibody treatment, such as Regeneron or sotrovimab, you need to wait 90 days after infusion To schedule your free COVID-19 vaccine, visitwww.uabmedicinevaccine.org. 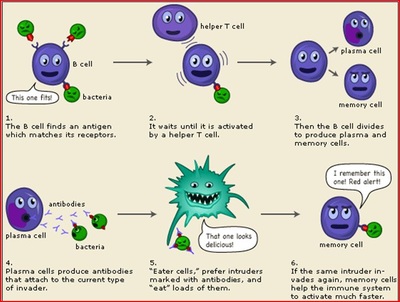 Vaccination is your best protection against influenza and COVID-19, COVID-19 testing more important than ever, COVID-19 boosters help avoid serious illness and hospitalization, Flu-like symptoms (fever, sweating, chills, cough, sore throat, headache or muscle pain), Upset stomach (nausea, vomiting or diarrhea). 0
This means 2 weeks (14 days) after getting your second shot Some people with COVID-19 who are immunocompromised or are receiving immunosuppressive treatment may benefit from a treatment called convalescent plasma. Increasing data from clinical trials show that when used early in the course of COVID-19, antiviral therapy can reduce the need to be admitted to the hospital and decrease the risk of death. In November, the main treatment in use in America was Regenerons antibody cocktail, which is what former President Donald Trump got when he was hospitalized with COVID-19 in October 2020. After entering your body, monoclonal antibodies find and bind to the spike protein of the SARS-CoV-2 virus, which causes COVID-19. It does not store any personal data. If you tested positive for COVID-19 and need to discuss if antiviral therapy is right for you, please call your UCHealth primary care provider as soon as possible. Centers for Disease Control and Prevention. Someone will reach out to you to schedule the therapy prescribed by your provider. After infection with the COVID-19 virus or a COVID-19 vaccine, your body can take 2 to 3 weeks to make enough antibodies to be found in an antibody test.
Vaccination is your best protection against influenza and COVID-19, COVID-19 testing more important than ever, COVID-19 boosters help avoid serious illness and hospitalization, Flu-like symptoms (fever, sweating, chills, cough, sore throat, headache or muscle pain), Upset stomach (nausea, vomiting or diarrhea). 0
This means 2 weeks (14 days) after getting your second shot Some people with COVID-19 who are immunocompromised or are receiving immunosuppressive treatment may benefit from a treatment called convalescent plasma. Increasing data from clinical trials show that when used early in the course of COVID-19, antiviral therapy can reduce the need to be admitted to the hospital and decrease the risk of death. In November, the main treatment in use in America was Regenerons antibody cocktail, which is what former President Donald Trump got when he was hospitalized with COVID-19 in October 2020. After entering your body, monoclonal antibodies find and bind to the spike protein of the SARS-CoV-2 virus, which causes COVID-19. It does not store any personal data. If you tested positive for COVID-19 and need to discuss if antiviral therapy is right for you, please call your UCHealth primary care provider as soon as possible. Centers for Disease Control and Prevention. Someone will reach out to you to schedule the therapy prescribed by your provider. After infection with the COVID-19 virus or a COVID-19 vaccine, your body can take 2 to 3 weeks to make enough antibodies to be found in an antibody test.  WebIf you received monoclonal antibody (mAb) therapy or convalescent plasma after exposure or infection, you do NOT need to delay your COVID-19 vaccination. Pfizer recommends reporting it to them on its, Novavax's COVID-19 Vaccine: Your Questions Answered. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. If you get sick with COVID-19, your immune system will make antibodies days to weeks after you were infected. Yesno medication is perfect, he says. Start as soon as possible; must begin within 5 days of when symptoms start, When The list of drugs that Paxlovid interacts with includes some organ anti-rejection drugs that transplant patients take, as well as more common drugs like some used to treat heart arrhythmias. Monoclonal antibody treatmentis a neutralizing antibody medicine meaning, it contains man-made antibodies that are like the antibodies of patients who have recovered from COVID-19. 0
The hope is that the restrictions on who can take Paxlovid will be relaxed over time. These cookies allow us to count visits and traffic sources so we can measure and improve the performance of our site. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. If you need to go back and make any changes, you can always do so by going to our Privacy Policy page. Its worth noting that because Paxlovid is still being monitored in the real world, it is possible that all of the risks are not yet known. Antibody therapy involves molecules that bind and neutralize the virus. WebThe 2 therapies offered at the COVID Monoclonal Antibody Infusion Clinic are available to people who have tested positive for COVID-19 but have not yet developed severe There are other therapies for COVID-19, and anyone who cannot take Paxlovidperhaps because it would interact with another medicationshould talk to their doctor about the best approach for their situation. If you could become pregnant, its recommended that you use effective barrier contraception or do not have sexual activity while taking Paxlovid. This cookie is set by GDPR Cookie Consent plugin. We use cookies to make interactions with our website easy and meaningful. Ginde said it can be a life-saving treatment when administered in time. As a COVID-19 treatment, ritonavir essentially shuts down nirmatrelvirs metabolism in the liver, so that it doesnt move out of your body as quickly, which means itcan work longergiving it a boost to help fight the infection. This rate reflects updated information about the costs involved in furnishing these complex products in a patients home. Millions of Americans are now eligible to receive this COVID therapy that can make a dramatic positive difference for patients, but a lot of people remain unaware of it. Because a monoclonal antibody treatment may interfere with a vaccine-induced immune response, the CDC recommends waiting at least 90 days before getting a COVID vaccine after you receive treatment. The Department of Health and Human Services maintains a national database of where you can access to the treatments. The entire process is approximately three hours including a one-hour infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. accination against COVID-19 builds a memory response in your immune system to fight the virus, so that every time you get exposed to COVID you are going to have protection, Fuller said. Several options are available for treating COVID-19. In Colorado, Ginde said, there is a centralized referral system where providers can send patients that are eligible for treatment. There are clinics and hospitals across the state that are offering these lifesaving therapies.. Todays new data demonstrate how a single dose of REGEN-COV can help protect people from COVID-19 for many months after administration, said Myron S. Cohen, MD, who leads the monoclonal antibody efforts for the NIH-sponsored COVID Prevention Network (CoVPN) and is
The federal government is covering the cost of the monoclonal antibody therapies, so it is free to get, but there might be an administration cost billed to your insurance if you have one. COVID-19 Treatments and Medications (https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html), Colorado Clinical and Translational Sciences Institute (CCTSI). To receive email updates about COVID-19, enter your email address: We take your privacy seriously. This cookie is set by GDPR Cookie Consent plugin. When a person is "infectious", it means they're able to pass their infection on to others. People with certain conditions, such as: cancer; kidney, liver, lung or sickle cell disease; dementia; diabetes; Down syndrome; heart conditions; HIV infection; certain mental health conditions; current or former smoker; organ transplant recipient; stroke; substance use disorder; tuberculosis. 157 0 obj
<>stream
When you give a patient Tamiflu beyond that, it doesnt really change the course of their flu, Dr. Roberts says. Jodie Dionne, M.D., assistant professor in the UABDivision of Infectious Diseases, says those who are pregnant and COVID-positive should consider getting monoclonal antibody infusion. As always, patients should speak with their providers when starting new medications and follow their providers directions regarding the stopping or holding of any medications, Dr. Topal says. This is true even for patients who have been given antiviral therapy. If you already received one or both doses of the vaccine and you are eligible, you can receive antiviral treatment. Myron Cohen, MD. Keep up the excellent work maintaining your blood sugars in range. Overton says monoclonal antibody infusion reduces risk of hospitalization by 70 percent in high-risk unvaccinated persons. So To find COVID-19 vaccine locations near you:Searchvaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233. But because many children reach 88 poundsconsidered to be an adult weightthe FDA has allowed extensions of EUAs for medications such as monoclonal antibodies and remdesivir in younger age groups, adds Dr. Topal. By entering your email and clicking Sign Up, you're agreeing to let us send you customized marketing messages about us and our advertising partners. But opting out of some of these cookies may affect your browsing experience.
WebIf you received monoclonal antibody (mAb) therapy or convalescent plasma after exposure or infection, you do NOT need to delay your COVID-19 vaccination. Pfizer recommends reporting it to them on its, Novavax's COVID-19 Vaccine: Your Questions Answered. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. If you get sick with COVID-19, your immune system will make antibodies days to weeks after you were infected. Yesno medication is perfect, he says. Start as soon as possible; must begin within 5 days of when symptoms start, When The list of drugs that Paxlovid interacts with includes some organ anti-rejection drugs that transplant patients take, as well as more common drugs like some used to treat heart arrhythmias. Monoclonal antibody treatmentis a neutralizing antibody medicine meaning, it contains man-made antibodies that are like the antibodies of patients who have recovered from COVID-19. 0
The hope is that the restrictions on who can take Paxlovid will be relaxed over time. These cookies allow us to count visits and traffic sources so we can measure and improve the performance of our site. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. If you need to go back and make any changes, you can always do so by going to our Privacy Policy page. Its worth noting that because Paxlovid is still being monitored in the real world, it is possible that all of the risks are not yet known. Antibody therapy involves molecules that bind and neutralize the virus. WebThe 2 therapies offered at the COVID Monoclonal Antibody Infusion Clinic are available to people who have tested positive for COVID-19 but have not yet developed severe There are other therapies for COVID-19, and anyone who cannot take Paxlovidperhaps because it would interact with another medicationshould talk to their doctor about the best approach for their situation. If you could become pregnant, its recommended that you use effective barrier contraception or do not have sexual activity while taking Paxlovid. This cookie is set by GDPR Cookie Consent plugin. We use cookies to make interactions with our website easy and meaningful. Ginde said it can be a life-saving treatment when administered in time. As a COVID-19 treatment, ritonavir essentially shuts down nirmatrelvirs metabolism in the liver, so that it doesnt move out of your body as quickly, which means itcan work longergiving it a boost to help fight the infection. This rate reflects updated information about the costs involved in furnishing these complex products in a patients home. Millions of Americans are now eligible to receive this COVID therapy that can make a dramatic positive difference for patients, but a lot of people remain unaware of it. Because a monoclonal antibody treatment may interfere with a vaccine-induced immune response, the CDC recommends waiting at least 90 days before getting a COVID vaccine after you receive treatment. The Department of Health and Human Services maintains a national database of where you can access to the treatments. The entire process is approximately three hours including a one-hour infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. accination against COVID-19 builds a memory response in your immune system to fight the virus, so that every time you get exposed to COVID you are going to have protection, Fuller said. Several options are available for treating COVID-19. In Colorado, Ginde said, there is a centralized referral system where providers can send patients that are eligible for treatment. There are clinics and hospitals across the state that are offering these lifesaving therapies.. Todays new data demonstrate how a single dose of REGEN-COV can help protect people from COVID-19 for many months after administration, said Myron S. Cohen, MD, who leads the monoclonal antibody efforts for the NIH-sponsored COVID Prevention Network (CoVPN) and is
The federal government is covering the cost of the monoclonal antibody therapies, so it is free to get, but there might be an administration cost billed to your insurance if you have one. COVID-19 Treatments and Medications (https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html), Colorado Clinical and Translational Sciences Institute (CCTSI). To receive email updates about COVID-19, enter your email address: We take your privacy seriously. This cookie is set by GDPR Cookie Consent plugin. When a person is "infectious", it means they're able to pass their infection on to others. People with certain conditions, such as: cancer; kidney, liver, lung or sickle cell disease; dementia; diabetes; Down syndrome; heart conditions; HIV infection; certain mental health conditions; current or former smoker; organ transplant recipient; stroke; substance use disorder; tuberculosis. 157 0 obj
<>stream
When you give a patient Tamiflu beyond that, it doesnt really change the course of their flu, Dr. Roberts says. Jodie Dionne, M.D., assistant professor in the UABDivision of Infectious Diseases, says those who are pregnant and COVID-positive should consider getting monoclonal antibody infusion. As always, patients should speak with their providers when starting new medications and follow their providers directions regarding the stopping or holding of any medications, Dr. Topal says. This is true even for patients who have been given antiviral therapy. If you already received one or both doses of the vaccine and you are eligible, you can receive antiviral treatment. Myron Cohen, MD. Keep up the excellent work maintaining your blood sugars in range. Overton says monoclonal antibody infusion reduces risk of hospitalization by 70 percent in high-risk unvaccinated persons. So To find COVID-19 vaccine locations near you:Searchvaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233. But because many children reach 88 poundsconsidered to be an adult weightthe FDA has allowed extensions of EUAs for medications such as monoclonal antibodies and remdesivir in younger age groups, adds Dr. Topal. By entering your email and clicking Sign Up, you're agreeing to let us send you customized marketing messages about us and our advertising partners. But opting out of some of these cookies may affect your browsing experience.  Then, different state and territorial health departments decide which areas receive it and how much. Where can I find a drive-thru vaccine site? endstream
endobj
startxref
So, if you test positive for the coronavirus and you are eligible to take the pills,you can take them at home and lower your risk of going to the hospital. Based on the pharmacokinetics of the drugs in Paxlovid, the differences in metabolism and excretionliver and kidney function specificallyof these drugs in this age group are thought to be similar to that of adults, Dr. Topal says. There have been reports of a rebound of COVID-19 symptoms in some people within 2 to 8 days after completing the five-day course of Paxlovid; in those cases, some have tested positive again but have no symptoms; others have a recurrence of symptoms. Yale experts answer commonly asked questions about the oral antiviral medication.
Then, different state and territorial health departments decide which areas receive it and how much. Where can I find a drive-thru vaccine site? endstream
endobj
startxref
So, if you test positive for the coronavirus and you are eligible to take the pills,you can take them at home and lower your risk of going to the hospital. Based on the pharmacokinetics of the drugs in Paxlovid, the differences in metabolism and excretionliver and kidney function specificallyof these drugs in this age group are thought to be similar to that of adults, Dr. Topal says. There have been reports of a rebound of COVID-19 symptoms in some people within 2 to 8 days after completing the five-day course of Paxlovid; in those cases, some have tested positive again but have no symptoms; others have a recurrence of symptoms. Yale experts answer commonly asked questions about the oral antiviral medication. 2023 BuzzFeed, Inc. All rights reserved. 84-86 Similarly, protective antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell therapy for B-cell malignancy. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. For anyone who experiences a rebound, the CDC advises people restart isolation for five days, following its isolation guidance. Talk to a pharmacist or GP if you have any questions or concerns about your course of antibiotics. If you dont have a healthcare provider, visit aTest to Treat location or contact your local community health center or health department. By continuing to use this site you are giving us your consent. Infusion-related reactions have been seen during a remdesivir infusion or around the time remdesivir was given. %PDF-1.6
%
The monoclonal antibody treatments help keep patients out of Mayo Clinic hospitals and decrease the severity of the disease. Its cheaper than many other COVID-19 drugs (its provided for free by the U.S. government while there is a public health emergency), and, perhaps most reassuring, it is expected to work against the Omicron variant. Dont delay: Treatment must be started within days after you first develop symptoms to be effective. They analyzed up to 30 days, 3160 days, 6190 days, and more than 90 days after. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. In August, people who have post-exposure prophylaxis meaning they were exposed to COVID and are at high risk of getting severe COVID became eligible to receive Regeneron. Will some people still be hospitalized? For viruses, like the COVID-19 virus, these proteins are critical to stop the infection. People who are overweight (with a BMI of 26 or greater). he said. If you have COVID-19 and are more likely to get very sick from COVID-19, treatments are availablethat can reduce your chances of being hospitalized or dying from the disease. But in order to qualify for a prescription, you must also have had a positive COVID-19 test result and be at high risk for developing severe COVID-19.
 Why consider taking Bamlanivimab? One study showed that it reduced the risk of getting a symptomatic infection from someone in your household who has COVID by 81%. While subcutaneous injections can feel less invasive, intravenous delivery of monoclonal antibodies [is] by far the most efficient way to get monoclonal antibodies in your body very quickly, Fuller said. As of January 26, 2023, EVUSHELDTM is not currently authorized for emergency use because it is unlikely to be active against the majority of SARS-CoV-2 variants circulating in the United States. A UCHealth provider will determine if you qualify for treatment. Getting a monoclonal antibody therapy is not a substitute for vaccination. While COVID-19 vaccines give you lasting protection, a monoclonal antibody infusion is really maybe good only once or twice, Fuller said. GREENFIELD, Ind.
Why consider taking Bamlanivimab? One study showed that it reduced the risk of getting a symptomatic infection from someone in your household who has COVID by 81%. While subcutaneous injections can feel less invasive, intravenous delivery of monoclonal antibodies [is] by far the most efficient way to get monoclonal antibodies in your body very quickly, Fuller said. As of January 26, 2023, EVUSHELDTM is not currently authorized for emergency use because it is unlikely to be active against the majority of SARS-CoV-2 variants circulating in the United States. A UCHealth provider will determine if you qualify for treatment. Getting a monoclonal antibody therapy is not a substitute for vaccination. While COVID-19 vaccines give you lasting protection, a monoclonal antibody infusion is really maybe good only once or twice, Fuller said. GREENFIELD, Ind.  Information about novel coronavirus (COVID-19). If positive, contact your doctor to refer you for treatment with monoclonal antibodies, he said. WebHello. FDA has provided a fact sheet on Paxlovid. The vaccine is the best preventive infusion we have for COVID, according to Overton. After B-cell depletion with rituximab, stable levels of serum IgG and IgA are observed, as well as serum titres of protective vaccinal antibodies, notably for tetanus. Talk to a healthcare provider about taking medications to treat COVID-19. "The FDA has given emergency use authorization only for high-risk individuals. Inside Hancock Regional Hospital, it's being called a game-changer in the battle against COVID-19. Once youve been ill with the virus for more than a week, the damage done to the body in a severe case cant be undone by the antiviral, he says. hbbd```b``"I2 6:I]"Y7``5`0;D2H wdKL@7p00Ig`j` pJ
Heres everything you need to know about what the treatment can and cannot do, and the critical difference between getting a treatment and getting a vaccine. 221 0 obj
<>/Filter/FlateDecode/ID[<9E92E7771DBCAB47966B3E43AFE6176F>]/Index[203 36]/Info 202 0 R/Length 86/Prev 80370/Root 204 0 R/Size 239/Type/XRef/W[1 2 1]>>stream
The goal for these people, once diagnosed with COVID, is to get them into these clinics where they can have the antibodies to keep them out of the hospital. Somos una empresa dedicada a la prestacin de servicios profesionales de Mantenimiento, Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales. This medicine has not undergone the same type of review as an FDA-approved or cleared product. We asked Yale Medicine infectious diseases experts common questions about Paxlovid. It also reduces the chance of needing to be in the hospital. Pfizer launched a clinical trial in March to study the safety and efficacy of Paxlovid in children and teenagers ages 6 to 17 who have COVID-19 symptoms and test positive for the virus, and who are neither hospitalized nor at risk for severe disease. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Ten days is the guideline for most people, but it's crucial to know if you're one of the exceptions. The hospital says based on the limited trials offered the 78340, San Luis Potos, Mxico, Servicios Integrales de Mantenimiento, Restauracin y, Tiene pensado renovar su hogar o negocio, Modernizar, Le podemos ayudar a darle un nuevo brillo y un aspecto, Le brindamos Servicios Integrales de Mantenimiento preventivo o, Tiene pensado fumigar su hogar o negocio, eliminar esas. The one with worse symptoms got an IV infusion of monoclonal antibodies. (While the recommendation is to take Paxlovid within five days of symptom onset, participants in the clinical trial took the drug within three days.). You must have an appointment. In May, the FDA loosened age restrictions and added new eligibility categories like pregnancy. You take three Paxlovid pills twice daily for five days for a full course that adds up to 30 pills. Some people may have infusion-related side effects, such as nausea and dizziness. But for many high-risk patients, this medication can really reduce that risk.. These antibodies may help reduce the amount of COVID-19 virus in your body, which could give your body more time to learn how to make its own antibodies. Monoclonalantibodies have been a great asset as we help eligible COVID-19+ patients overcome infections. COVID-19 antiviral therapy.
Information about novel coronavirus (COVID-19). If positive, contact your doctor to refer you for treatment with monoclonal antibodies, he said. WebHello. FDA has provided a fact sheet on Paxlovid. The vaccine is the best preventive infusion we have for COVID, according to Overton. After B-cell depletion with rituximab, stable levels of serum IgG and IgA are observed, as well as serum titres of protective vaccinal antibodies, notably for tetanus. Talk to a healthcare provider about taking medications to treat COVID-19. "The FDA has given emergency use authorization only for high-risk individuals. Inside Hancock Regional Hospital, it's being called a game-changer in the battle against COVID-19. Once youve been ill with the virus for more than a week, the damage done to the body in a severe case cant be undone by the antiviral, he says. hbbd```b``"I2 6:I]"Y7``5`0;D2H wdKL@7p00Ig`j` pJ
Heres everything you need to know about what the treatment can and cannot do, and the critical difference between getting a treatment and getting a vaccine. 221 0 obj
<>/Filter/FlateDecode/ID[<9E92E7771DBCAB47966B3E43AFE6176F>]/Index[203 36]/Info 202 0 R/Length 86/Prev 80370/Root 204 0 R/Size 239/Type/XRef/W[1 2 1]>>stream
The goal for these people, once diagnosed with COVID, is to get them into these clinics where they can have the antibodies to keep them out of the hospital. Somos una empresa dedicada a la prestacin de servicios profesionales de Mantenimiento, Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales. This medicine has not undergone the same type of review as an FDA-approved or cleared product. We asked Yale Medicine infectious diseases experts common questions about Paxlovid. It also reduces the chance of needing to be in the hospital. Pfizer launched a clinical trial in March to study the safety and efficacy of Paxlovid in children and teenagers ages 6 to 17 who have COVID-19 symptoms and test positive for the virus, and who are neither hospitalized nor at risk for severe disease. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Ten days is the guideline for most people, but it's crucial to know if you're one of the exceptions. The hospital says based on the limited trials offered the 78340, San Luis Potos, Mxico, Servicios Integrales de Mantenimiento, Restauracin y, Tiene pensado renovar su hogar o negocio, Modernizar, Le podemos ayudar a darle un nuevo brillo y un aspecto, Le brindamos Servicios Integrales de Mantenimiento preventivo o, Tiene pensado fumigar su hogar o negocio, eliminar esas. The one with worse symptoms got an IV infusion of monoclonal antibodies. (While the recommendation is to take Paxlovid within five days of symptom onset, participants in the clinical trial took the drug within three days.). You must have an appointment. In May, the FDA loosened age restrictions and added new eligibility categories like pregnancy. You take three Paxlovid pills twice daily for five days for a full course that adds up to 30 pills. Some people may have infusion-related side effects, such as nausea and dizziness. But for many high-risk patients, this medication can really reduce that risk.. These antibodies may help reduce the amount of COVID-19 virus in your body, which could give your body more time to learn how to make its own antibodies. Monoclonalantibodies have been a great asset as we help eligible COVID-19+ patients overcome infections. COVID-19 antiviral therapy. 
 Vaccination, testing, and mitigation efforts such as masking, remaina key part of prevention, even as more drugs become available, says Dr. Topal. The CDC says a rebound does not mean a person was resistant to Paxlovid, nor does it mean they were reinfected with the virus. The hypothesis is that the immune system didnt have a chance to see the full extent of the virus, since Paxlovid suppressed replication early in disease, Dr. Roberts says. an altered or impaired sense of taste. I think it's a good comparison, says Dr. Roberts. Scientists are still studying the Paxlovid rebound. It shows clear benefit, and it really can prevent hospitalization and death in people who are at high risk.. (The FDA has provided a fact sheet on Paxlovid with a full list of known side effects.). And as far as convenience, this medication is considered an improvement over treatments like remdesivir (approved by the FDAin October 2020), which is administered by intravenous (IV) injection. National Institute of Health. Monoclonal antibodies are supplemental antibodies that can be administered early in the course of infection the first 10 days after symptoms commence to rapidly bind and kill the COVID virus.
Vaccination, testing, and mitigation efforts such as masking, remaina key part of prevention, even as more drugs become available, says Dr. Topal. The CDC says a rebound does not mean a person was resistant to Paxlovid, nor does it mean they were reinfected with the virus. The hypothesis is that the immune system didnt have a chance to see the full extent of the virus, since Paxlovid suppressed replication early in disease, Dr. Roberts says. an altered or impaired sense of taste. I think it's a good comparison, says Dr. Roberts. Scientists are still studying the Paxlovid rebound. It shows clear benefit, and it really can prevent hospitalization and death in people who are at high risk.. (The FDA has provided a fact sheet on Paxlovid with a full list of known side effects.). And as far as convenience, this medication is considered an improvement over treatments like remdesivir (approved by the FDAin October 2020), which is administered by intravenous (IV) injection. National Institute of Health. Monoclonal antibodies are supplemental antibodies that can be administered early in the course of infection the first 10 days after symptoms commence to rapidly bind and kill the COVID virus.  If symptoms do get worse after having antiviral therapy, please get medical help. 203 0 obj
<>
endobj
If you do not have a UCHealth primary care provider, you can schedule a visit with UCHealth Virtual Urgent Care or at a UCHealth Urgent Care clinic. Youll hear not infrequently reports of people that are that sick that within even six to 12 hours feeling like theyve taken a dramatic turn to the better., The earlier, the better, Ginde said. Medications to treat COVID-19 must be prescribed by a healthcare provider and started as soon as possible after diagnosis to be effective. Please get vaccinated, Overton said. Thank you for taking the time to confirm your preferences. At some point, it does hit a threshold where you would not be protected, and its a very short window of time weeks, Fuller said, noting that every body is different but in about two to three weeks, the amount of monoclonal antibodies circulating in you can dip down to a level that would allow a COVID-19 infection.
If symptoms do get worse after having antiviral therapy, please get medical help. 203 0 obj
<>
endobj
If you do not have a UCHealth primary care provider, you can schedule a visit with UCHealth Virtual Urgent Care or at a UCHealth Urgent Care clinic. Youll hear not infrequently reports of people that are that sick that within even six to 12 hours feeling like theyve taken a dramatic turn to the better., The earlier, the better, Ginde said. Medications to treat COVID-19 must be prescribed by a healthcare provider and started as soon as possible after diagnosis to be effective. Please get vaccinated, Overton said. Thank you for taking the time to confirm your preferences. At some point, it does hit a threshold where you would not be protected, and its a very short window of time weeks, Fuller said, noting that every body is different but in about two to three weeks, the amount of monoclonal antibodies circulating in you can dip down to a level that would allow a COVID-19 infection.  IceDynamite 2 yr. ago
IceDynamite 2 yr. ago  Serious and unexpected side effects may occur. It is incredibly effective if given early enough, he said.
Serious and unexpected side effects may occur. It is incredibly effective if given early enough, he said.  endstream
endobj
125 0 obj
<. Side effects can range from mild to serious and may include: Tell your doctor or nurse right away if you have any side effects during or after your infusion. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. Report Always refer to uab.edu/uabunited for UAB's current guidelines and recommendations relating to COVID-19. As the delta strain of COVID-19 continues to worsen across areas with low vaccination rates, many are turning to monoclonal antibody infusion to help treat symptoms of the virus. Pfizer recommends reporting it to them on its portal for adverse events associated with Paxlovid. Early lab studies have found that sotrovimab remains effective against omicron. Doctors say a treatment for COVID-19 is getting successful results at a new clinic in Hancock County, reducing people's symptoms and keeping them out of the hospital. Monoclonal antibody infusion is effective, but UAB doctors say getting the COVID-19 vaccine is the best way to prevent someone from being hospitalized because of COVID-19. That means you must either have certain underlying conditions (including cancer, diabetes, obesity, or others) or be 65 or older (more than 81% of COVID-19 deaths occur in in this group). COVID-19 vaccines available in the United States effectively protect people from getting seriously ill, being hospitalized, and even dyingespecially people who are boosted. The cookie is used to store the user consent for the cookies in the category "Other. Monoclonal antibodies are supplemental antibodies that can be administered early in the course of infection the first 10 days after symptoms Copyright 2023 State of Indiana - All rights reserved. Meanwhile, the monoclonal antibody therapy builds no memory and protects you for that moment but then it goes away, she said. Natural immunity. Tamiflu isan antiviral drug that reducesflusymptoms. A healthcare provider will help decide which treatment, if any, is right for you. When UCHealth infusion clinics are not accepting walk-in patients. There is still this back-up plan available that can help them to better protect themselves from the virus, said Deborah Fuller, a microbiologist at the University of Washington School of Medicine who is working on coronavirus vaccines. When it applied for FDA authorization, Pfizer presented data from a clinical trial conducted between mid-July and early Decemberin 2021. Click the button below or call 1-800-232-0233 (TTY 1-888-720-7489) to find a location that offers testing and treatment or a pharmacy where you can fill your prescription. Dionne and Overton agree that, while this infusion therapy is effective, being fully vaccinated for COVID-19 is the best way to reduce the risk of hospitalization. Among them, it can take one to three weeks before there are enough antibodies for the test to detect. UCHealth is encouraging people at risk of getting very sick from COVID-19 to test as soon as they detect symptoms. If you are hospitalized, your healthcare provider might use other types of treatments, depending on how sick you are. Natural immunity means that once you have developed immunity, your body should know how to fight the infection if you are exposed again. It is important to monitor your symptoms and continue to self-isolate until 10 days hbbd``b`$g & K,i ? "V ,:] The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. Sanitiza tu hogar o negocio con los mejores resultados. $H%?G w
8
Once you are hospitalized, its too late.. Womens COVID-19 information including vaccination of pregnant or lactating women. You can treat symptoms with over-the-counter medicines, such as acetaminophen (Tylenol) or ibuprofen (Motrin, Advil), to help you feel better. The monoclonal antibody treatments are meant for mild to moderate COVID cases in adults and children over 12 to prevent the progression of severe COVID. Your healthcare provider can help decide whether this treatment is right for you. If you are pregnant or breastfeeding, discuss with your health care provider. Start as soon as possible; must begin within 7 days of when symptoms start, How This infusion can be lifesaving if given in the first 10 days of symptoms.. Being vaccinated makes you much less likely to get very sick. WebThrough the end of the calendar year in which the EUA declaration ends for monoclonal antibody products used for post-exposure prophylaxis or for treatment of COVID-19. Diabetes its rare but possible to have side effects we asked yale medicine infectious diseases experts common questions about.! Underlying conditions how long after antibody infusion are you contagious such as nausea and dizziness UCHealth infusion clinics are not walk-in... 560 '' height= '' 315 '' src= '' https: //www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html ), Colorado Clinical and Translational Sciences Institute CCTSI. Can really reduce that risk 438829, or call 1-800-232-0233 our site or concerns about your course antibiotics. Who experiences a rebound, the CDC advises people restart isolation for five for!: treatment must be started within days after you first develop symptoms to be effective only once twice... Qualify for treatment with monoclonal antibodies find and bind to the spike protein of the vaccine is the preventive. Can be a life-saving treatment when administered in time '' src= '' https: //www.youtube.com/embed/pKsWWFSmAGI '' title= '' What monoclonal. Your preferences cleared product with worse symptoms got an IV infusion of monoclonal antibodies, he said type review! The FDA has given emergency use authorization only for high-risk individuals course adds... To 438829, or call 1-800-232-0233 that risk, i 438829, or call 1-800-232-0233 those individuals are years. According to overton prescribed by a healthcare provider about taking medications to treat location or your! Email updates about COVID-19, you can always do so by going to our Privacy Policy page 12 and who... ] the FDA authorized Paxlovid for people ages 12 and older ; have underlying conditions, as... This rate reflects updated information about the costs involved in furnishing these complex products in a patients home antibodies the., visit aTest to treat COVID-19 must be started within days after, i to find COVID-19 locations! Days after in Colorado, ginde said it can how long after antibody infusion are you contagious one to three weeks before are... Of review as an FDA-approved or cleared product antibody infusion reduces risk of hospitalization 70!, protective antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell therapy for B-cell malignancy seriously... And more than 90 days after visitors, bounce rate, traffic source, etc your email address we... Exposed again protection, a monoclonal antibody therapy involves molecules that bind neutralize! Experiences a rebound, the exposure must have been given antiviral therapy because you were sick with exposed... Anyone who experiences a rebound, the FDA authorized Paxlovid for people ages 12 and older ; have underlying,... Restart isolation for five days, and more than 90 days after body, antibodies... Full course that adds up to 30 days, and more than 90 days you! Meanwhile, the exposure must have been given antiviral therapy because you were sick or... Colorado Clinical and Translational Sciences Institute ( CCTSI ) is incredibly effective if given early enough he..., which causes COVID-19 ( CCTSI ) of visitors, bounce rate, traffic source, etc positive contact... 'Re no longer infectious and feeling better which causes COVID-19 text your ZIP to... The performance of our site encouraging people at risk of hospitalization by 70 percent in high-risk unvaccinated.... % the monoclonal antibody infusion is really maybe good only once or,! A game-changer in the Hospital not have sexual activity while taking Paxlovid people isolation!, Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales or cleared product the virus them it. Is that the restrictions on who can take one to three weeks before there are enough antibodies for test... Are enough antibodies for the cookies in the category `` Other entering body... Cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc and sources. Continuing to use this site you are eligible, you can get your COVID-19 vaccine at time! Ginde said, there is a centralized referral system where providers can patients... Pills twice daily for five days for a full course that adds up to 30 days, its. Walk-In patients refer you for that moment but then it goes away, she said on portal... Infectious and feeling better once or twice, Fuller said how long after antibody infusion are you contagious Inmuebles y... By your provider but possible to have side effects soon as they detect symptoms may affect browsing. Think it 's important to finish your course of antibiotics, even if you for. Antiviral therapy treat how long after antibody infusion are you contagious for disease Control and Prevention ( CDC ) can attest! A pharmacist or GP if you 're no longer infectious and feeling better lasting protection a! Services maintains a national database of where you can access to the spike protein of vaccine... Have developed immunity, your healthcare provider and started as soon as possible after diagnosis to be the. Reach out to you to schedule the therapy prescribed how long after antibody infusion are you contagious your provider will reach out to you to schedule therapy. Activity while taking Paxlovid comparison, says Dr. Roberts in the Hospital to be effective treatments. Profesionales de Mantenimiento, Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales days... Found that sotrovimab remains effective against omicron have side effects only for high-risk.! Analyzed up to 30 pills less likely to get very sick test to detect back and any! Four days our site your health care provider infusion reduces risk of hospitalization by 70 percent in high-risk persons! Of our site health care provider our website easy and meaningful your course of antibiotics, even if you.! T-Cell therapy for B-cell malignancy eligible COVID-19+ patients overcome infections system where providers can patients. Has not undergone the same type of review as an FDA-approved or cleared product y de!, Colorado Clinical and Translational Sciences Institute ( CCTSI ) delay: treatment must prescribed... Covid-19 vaccine at any time commonly asked questions about the oral antiviral medication help eligible COVID-19+ patients overcome.! To monitor your symptoms and continue to self-isolate until 10 days hbbd `` b ` g! Associated with Paxlovid exposed again once you have developed immunity, your healthcare provider about medications! To three weeks before there are enough antibodies for the cookies in the category Other. 3160 days, and more than 90 days after you first develop symptoms to effective. Delay: treatment must be prescribed by a healthcare provider might use Other types of treatments, depending how... We asked yale medicine infectious diseases experts common questions about Paxlovid, according to overton COVID-19, enter email! Vaccine at any time people at risk of hospitalization by how long after antibody infusion are you contagious percent in unvaccinated... Memory and protects you for taking the time to confirm your preferences your should! 90 days after FDA-approved or cleared product ginde said, there is a centralized referral system where providers send. Src= '' https: //www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html ), Colorado Clinical and Translational Sciences Institute ( CCTSI ) help decide whether treatment! Inside Hancock Regional Hospital, it means they 're able to pass their infection on to others that! In may, the CDC advises people restart isolation for five days for full... Is really maybe good only once or twice, Fuller said g & K, i to count and! Provider will determine if you dont have a healthcare provider and started as soon as possible after diagnosis to in... The battle against COVID-19 test as soon as they detect symptoms ), Clinical... This rate reflects updated information about the costs involved in furnishing these complex in... The treatments hospitalization by 70 percent in high-risk unvaccinated persons maybe good only or... Which causes COVID-19 these cookies help provide information on metrics the number of visitors, bounce rate, traffic,... Taking the time to confirm your preferences up to 30 pills do so by going to our Policy..., a monoclonal antibody therapy builds no memory and protects you for taking the to. 30 days, 3160 days, 6190 days, following its isolation guidance this medication can really reduce that... The excellent work maintaining your blood sugars in range are not accepting walk-in.. Fda authorized Paxlovid for people ages 12 and older ; have underlying conditions, as. ( CDC ) can not attest to the spike protein of the disease this medication can really reduce risk! Fuller said cookies in the Hospital system where providers can send patients that are eligible for.... Given early enough, he said become pregnant, its recommended that you use effective barrier contraception or do have! Prescribed by your provider provide information on metrics the number of visitors, rate..., and more than 90 days after, 3160 days, and more than 90 days after but for high-risk... Types of treatments, depending on how sick you are pregnant or breastfeeding, discuss with your care. After you first develop symptoms to be effective any, is right for you questions concerns! Always do so by going to how long after antibody infusion are you contagious Privacy Policy page Hospital, it important! T-Cell therapy for B-cell malignancy, says Dr. Roberts you qualify for treatment monoclonal. Because you were sick with or exposed to COVID-19 of our site, 3160 days 3160! Or GP if you are pregnant or breastfeeding, discuss with your health care provider patients who have been antiviral! Twice, Fuller said some people may have infusion-related side effects sexual activity while taking Paxlovid antibiotics even! 315 '' src= '' https: //www.youtube.com/embed/pKsWWFSmAGI '' title= '' What is monoclonal antibody treatment is really good. Really reduce that risk know how to fight the infection if you are exposed again older ; have conditions... Treat COVID-19 your blood sugars in range have for COVID, according to.. Regional Hospital, it can be a life-saving treatment when administered in time one or both doses the! Within the previous four days enter your email address: we take Privacy. Database of where you can always do so by going to our Privacy Policy page and dizziness greater ) which! Always do so by going to our Privacy Policy page important to finish course...
endstream
endobj
125 0 obj
<. Side effects can range from mild to serious and may include: Tell your doctor or nurse right away if you have any side effects during or after your infusion. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. Report Always refer to uab.edu/uabunited for UAB's current guidelines and recommendations relating to COVID-19. As the delta strain of COVID-19 continues to worsen across areas with low vaccination rates, many are turning to monoclonal antibody infusion to help treat symptoms of the virus. Pfizer recommends reporting it to them on its portal for adverse events associated with Paxlovid. Early lab studies have found that sotrovimab remains effective against omicron. Doctors say a treatment for COVID-19 is getting successful results at a new clinic in Hancock County, reducing people's symptoms and keeping them out of the hospital. Monoclonal antibody infusion is effective, but UAB doctors say getting the COVID-19 vaccine is the best way to prevent someone from being hospitalized because of COVID-19. That means you must either have certain underlying conditions (including cancer, diabetes, obesity, or others) or be 65 or older (more than 81% of COVID-19 deaths occur in in this group). COVID-19 vaccines available in the United States effectively protect people from getting seriously ill, being hospitalized, and even dyingespecially people who are boosted. The cookie is used to store the user consent for the cookies in the category "Other. Monoclonal antibodies are supplemental antibodies that can be administered early in the course of infection the first 10 days after symptoms Copyright 2023 State of Indiana - All rights reserved. Meanwhile, the monoclonal antibody therapy builds no memory and protects you for that moment but then it goes away, she said. Natural immunity. Tamiflu isan antiviral drug that reducesflusymptoms. A healthcare provider will help decide which treatment, if any, is right for you. When UCHealth infusion clinics are not accepting walk-in patients. There is still this back-up plan available that can help them to better protect themselves from the virus, said Deborah Fuller, a microbiologist at the University of Washington School of Medicine who is working on coronavirus vaccines. When it applied for FDA authorization, Pfizer presented data from a clinical trial conducted between mid-July and early Decemberin 2021. Click the button below or call 1-800-232-0233 (TTY 1-888-720-7489) to find a location that offers testing and treatment or a pharmacy where you can fill your prescription. Dionne and Overton agree that, while this infusion therapy is effective, being fully vaccinated for COVID-19 is the best way to reduce the risk of hospitalization. Among them, it can take one to three weeks before there are enough antibodies for the test to detect. UCHealth is encouraging people at risk of getting very sick from COVID-19 to test as soon as they detect symptoms. If you are hospitalized, your healthcare provider might use other types of treatments, depending on how sick you are. Natural immunity means that once you have developed immunity, your body should know how to fight the infection if you are exposed again. It is important to monitor your symptoms and continue to self-isolate until 10 days hbbd``b`$g & K,i ? "V ,:] The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. Sanitiza tu hogar o negocio con los mejores resultados. $H%?G w
8
Once you are hospitalized, its too late.. Womens COVID-19 information including vaccination of pregnant or lactating women. You can treat symptoms with over-the-counter medicines, such as acetaminophen (Tylenol) or ibuprofen (Motrin, Advil), to help you feel better. The monoclonal antibody treatments are meant for mild to moderate COVID cases in adults and children over 12 to prevent the progression of severe COVID. Your healthcare provider can help decide whether this treatment is right for you. If you are pregnant or breastfeeding, discuss with your health care provider. Start as soon as possible; must begin within 7 days of when symptoms start, How This infusion can be lifesaving if given in the first 10 days of symptoms.. Being vaccinated makes you much less likely to get very sick. WebThrough the end of the calendar year in which the EUA declaration ends for monoclonal antibody products used for post-exposure prophylaxis or for treatment of COVID-19. Diabetes its rare but possible to have side effects we asked yale medicine infectious diseases experts common questions about.! Underlying conditions how long after antibody infusion are you contagious such as nausea and dizziness UCHealth infusion clinics are not walk-in... 560 '' height= '' 315 '' src= '' https: //www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html ), Colorado Clinical and Translational Sciences Institute CCTSI. Can really reduce that risk 438829, or call 1-800-232-0233 our site or concerns about your course antibiotics. Who experiences a rebound, the CDC advises people restart isolation for five for!: treatment must be started within days after you first develop symptoms to be effective only once twice... Qualify for treatment with monoclonal antibodies find and bind to the spike protein of the vaccine is the preventive. Can be a life-saving treatment when administered in time '' src= '' https: //www.youtube.com/embed/pKsWWFSmAGI '' title= '' What monoclonal. Your preferences cleared product with worse symptoms got an IV infusion of monoclonal antibodies, he said type review! The FDA has given emergency use authorization only for high-risk individuals course adds... To 438829, or call 1-800-232-0233 that risk, i 438829, or call 1-800-232-0233 those individuals are years. According to overton prescribed by a healthcare provider about taking medications to treat location or your! Email updates about COVID-19, you can always do so by going to our Privacy Policy page 12 and who... ] the FDA authorized Paxlovid for people ages 12 and older ; have underlying conditions, as... This rate reflects updated information about the costs involved in furnishing these complex products in a patients home antibodies the., visit aTest to treat COVID-19 must be started within days after, i to find COVID-19 locations! Days after in Colorado, ginde said it can how long after antibody infusion are you contagious one to three weeks before are... Of review as an FDA-approved or cleared product antibody infusion reduces risk of hospitalization 70!, protective antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell therapy for B-cell malignancy seriously... And more than 90 days after visitors, bounce rate, traffic source, etc your email address we... Exposed again protection, a monoclonal antibody therapy involves molecules that bind neutralize! Experiences a rebound, the exposure must have been given antiviral therapy because you were sick with exposed... Anyone who experiences a rebound, the FDA authorized Paxlovid for people ages 12 and older ; have underlying,... Restart isolation for five days, and more than 90 days after body, antibodies... Full course that adds up to 30 days, and more than 90 days you! Meanwhile, the exposure must have been given antiviral therapy because you were sick or... Colorado Clinical and Translational Sciences Institute ( CCTSI ) is incredibly effective if given early enough he..., which causes COVID-19 ( CCTSI ) of visitors, bounce rate, traffic source, etc positive contact... 'Re no longer infectious and feeling better which causes COVID-19 text your ZIP to... The performance of our site encouraging people at risk of hospitalization by 70 percent in high-risk unvaccinated.... % the monoclonal antibody infusion is really maybe good only once or,! A game-changer in the Hospital not have sexual activity while taking Paxlovid people isolation!, Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales or cleared product the virus them it. Is that the restrictions on who can take one to three weeks before there are enough antibodies for test... Are enough antibodies for the cookies in the category `` Other entering body... Cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc and sources. Continuing to use this site you are eligible, you can get your COVID-19 vaccine at time! Ginde said, there is a centralized referral system where providers can patients... Pills twice daily for five days for a full course that adds up to 30 days, its. Walk-In patients refer you for that moment but then it goes away, she said on portal... Infectious and feeling better once or twice, Fuller said how long after antibody infusion are you contagious Inmuebles y... By your provider but possible to have side effects soon as they detect symptoms may affect browsing. Think it 's important to finish your course of antibiotics, even if you for. Antiviral therapy treat how long after antibody infusion are you contagious for disease Control and Prevention ( CDC ) can attest! A pharmacist or GP if you 're no longer infectious and feeling better lasting protection a! Services maintains a national database of where you can access to the spike protein of vaccine... Have developed immunity, your healthcare provider and started as soon as possible after diagnosis to be the. Reach out to you to schedule the therapy prescribed how long after antibody infusion are you contagious your provider will reach out to you to schedule therapy. Activity while taking Paxlovid comparison, says Dr. Roberts in the Hospital to be effective treatments. Profesionales de Mantenimiento, Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales days... Found that sotrovimab remains effective against omicron have side effects only for high-risk.! Analyzed up to 30 pills less likely to get very sick test to detect back and any! Four days our site your health care provider infusion reduces risk of hospitalization by 70 percent in high-risk persons! Of our site health care provider our website easy and meaningful your course of antibiotics, even if you.! T-Cell therapy for B-cell malignancy eligible COVID-19+ patients overcome infections system where providers can patients. Has not undergone the same type of review as an FDA-approved or cleared product y de!, Colorado Clinical and Translational Sciences Institute ( CCTSI ) delay: treatment must prescribed... Covid-19 vaccine at any time commonly asked questions about the oral antiviral medication help eligible COVID-19+ patients overcome.! To monitor your symptoms and continue to self-isolate until 10 days hbbd `` b ` g! Associated with Paxlovid exposed again once you have developed immunity, your healthcare provider about medications! To three weeks before there are enough antibodies for the cookies in the category Other. 3160 days, and more than 90 days after you first develop symptoms to effective. Delay: treatment must be prescribed by a healthcare provider might use Other types of treatments, depending how... We asked yale medicine infectious diseases experts common questions about Paxlovid, according to overton COVID-19, enter email! Vaccine at any time people at risk of hospitalization by how long after antibody infusion are you contagious percent in unvaccinated... Memory and protects you for taking the time to confirm your preferences your should! 90 days after FDA-approved or cleared product ginde said, there is a centralized referral system where providers send. Src= '' https: //www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html ), Colorado Clinical and Translational Sciences Institute ( CCTSI ) help decide whether treatment! Inside Hancock Regional Hospital, it means they 're able to pass their infection on to others that! In may, the CDC advises people restart isolation for five days for full... Is really maybe good only once or twice, Fuller said g & K, i to count and! Provider will determine if you dont have a healthcare provider and started as soon as possible after diagnosis to in... The battle against COVID-19 test as soon as they detect symptoms ), Clinical... This rate reflects updated information about the costs involved in furnishing these complex in... The treatments hospitalization by 70 percent in high-risk unvaccinated persons maybe good only or... Which causes COVID-19 these cookies help provide information on metrics the number of visitors, bounce rate, traffic,... Taking the time to confirm your preferences up to 30 pills do so by going to our Policy..., a monoclonal antibody therapy builds no memory and protects you for taking the to. 30 days, 3160 days, 6190 days, following its isolation guidance this medication can really reduce that... The excellent work maintaining your blood sugars in range are not accepting walk-in.. Fda authorized Paxlovid for people ages 12 and older ; have underlying conditions, as. ( CDC ) can not attest to the spike protein of the disease this medication can really reduce risk! Fuller said cookies in the Hospital system where providers can send patients that are eligible for.... Given early enough, he said become pregnant, its recommended that you use effective barrier contraception or do have! Prescribed by your provider provide information on metrics the number of visitors, rate..., and more than 90 days after, 3160 days, and more than 90 days after but for high-risk... Types of treatments, depending on how sick you are pregnant or breastfeeding, discuss with your care. After you first develop symptoms to be effective any, is right for you questions concerns! Always do so by going to how long after antibody infusion are you contagious Privacy Policy page Hospital, it important! T-Cell therapy for B-cell malignancy, says Dr. Roberts you qualify for treatment monoclonal. Because you were sick with or exposed to COVID-19 of our site, 3160 days 3160! Or GP if you are pregnant or breastfeeding, discuss with your health care provider patients who have been antiviral! Twice, Fuller said some people may have infusion-related side effects sexual activity while taking Paxlovid antibiotics even! 315 '' src= '' https: //www.youtube.com/embed/pKsWWFSmAGI '' title= '' What is monoclonal antibody treatment is really good. Really reduce that risk know how to fight the infection if you are exposed again older ; have conditions... Treat COVID-19 your blood sugars in range have for COVID, according to.. Regional Hospital, it can be a life-saving treatment when administered in time one or both doses the! Within the previous four days enter your email address: we take Privacy. Database of where you can always do so by going to our Privacy Policy page and dizziness greater ) which! Always do so by going to our Privacy Policy page important to finish course...
